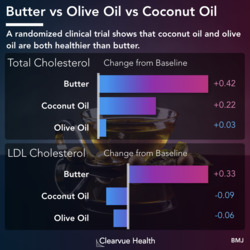
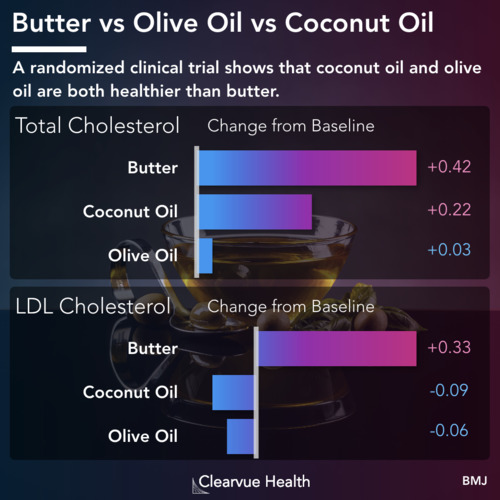
Why Migraines Matter
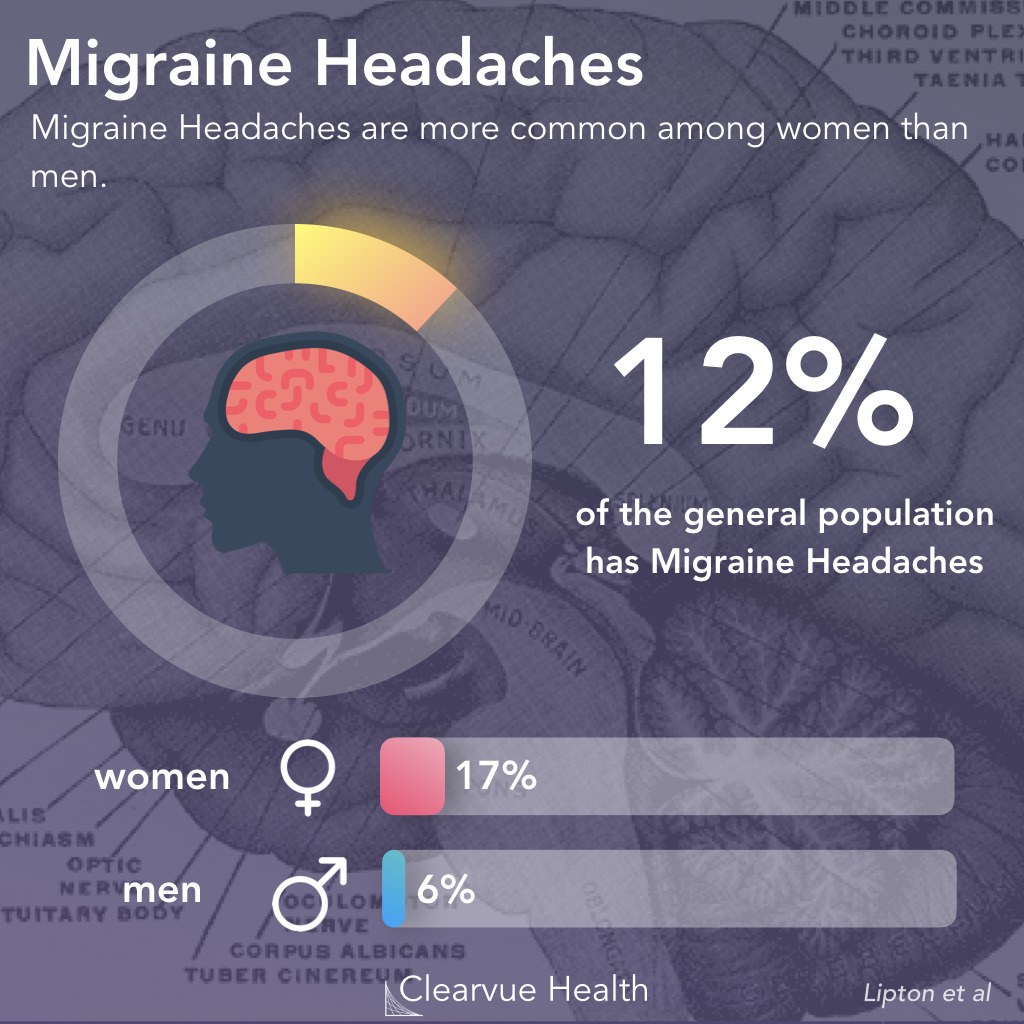
Figure 1: Migraine Epidemiology. Migraines are one of the most common medical conditions, affecting around 12% of the general population. It tends to be more common in women than in men. A study estimated that around 17% of women experience migraine headaches, while only 6% of men experience them.
Migraines are one of the most unpleasant conditions a person can face.
Migraines involve very painful headaches and other unpleasant symptoms such as sensitivity to light, sensitivity to sound, and nausea.
Migraine headaches affect 12% of the general population. For reasons still unknown, they are more common in women than in men.
There are currently no good treatments for migraines. Existing treatments do not work for most patients and they carry significant side effects.
A new generation of drugs seeks to address this issue with medications that target different and newly discovered mechanisms.
A recent phase III clinical trial, which is used to determine whether medication is effective and should be approved, was just published in the New England Journal of Medicine.
Source: Rimegepant, an Oral Calcitonin Gene–Related Peptide Receptor Antagonist, for Migraine
Phase 3 clinical trials are the largest of the 3 main phases of clinical trials. These are conducted typically after a medication has been shown to be safe and potentially effective. These trials are typically conducted for the purpose of determining whether a potential drug should be approved by the FDA.
Rimegepant and Migraine Pain
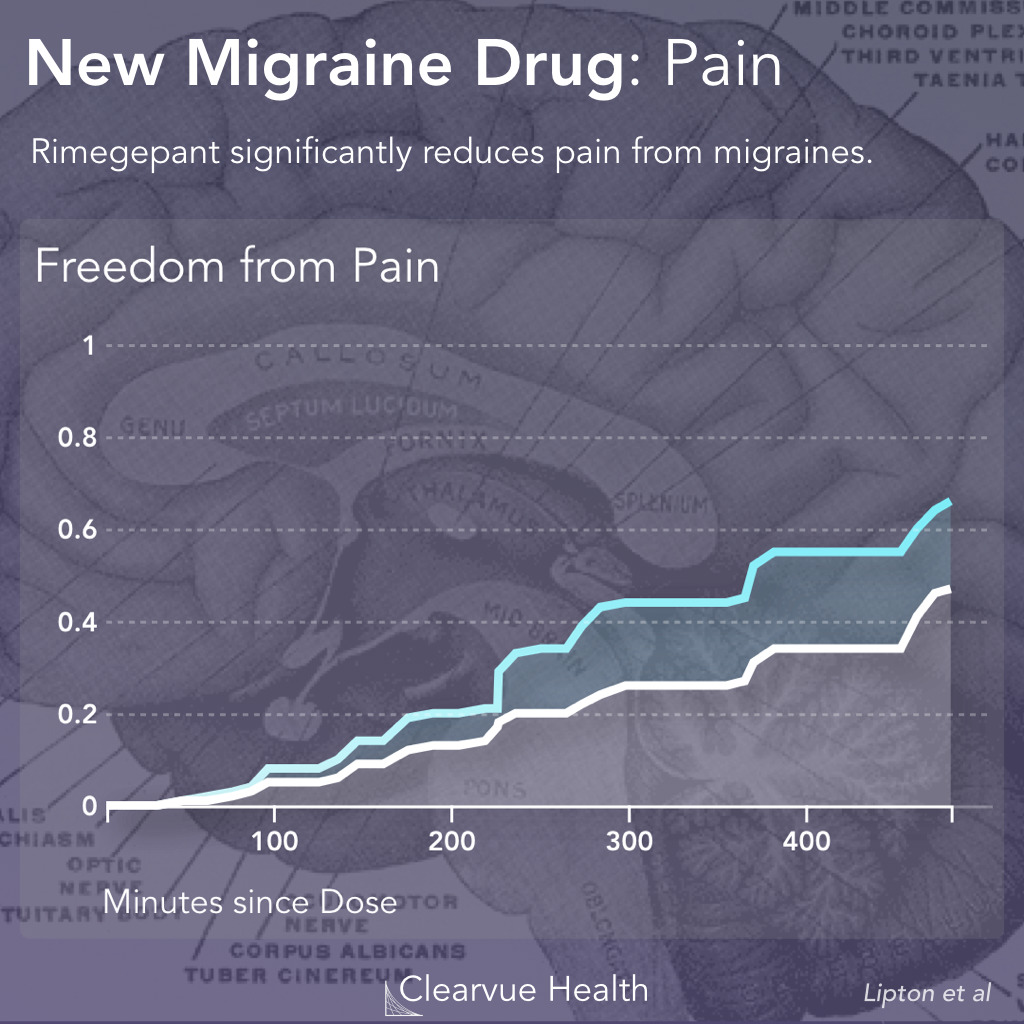
Figure 2: Rimegepant and Migraine Pain. Researchers tracked the pain in patients who took either rimonabant or a placebo control over the course of 520 minutes. They found that patients who took Rimegepant were more likely to experience freedom from pain than patients who took the placebo control. (19.6% vs 12.0%, p < 0.001)
The trial found that patients with took this medication had a significantly faster recovery from pain. However, as you can see in the chart above, this drug doesn’t stop the pain, it only helps speed up recovery.
Source: Rimegepant, an Oral Calcitonin Gene–Related Peptide Receptor Antagonist, for Migraine
Rimegepant and Migraine Symptoms
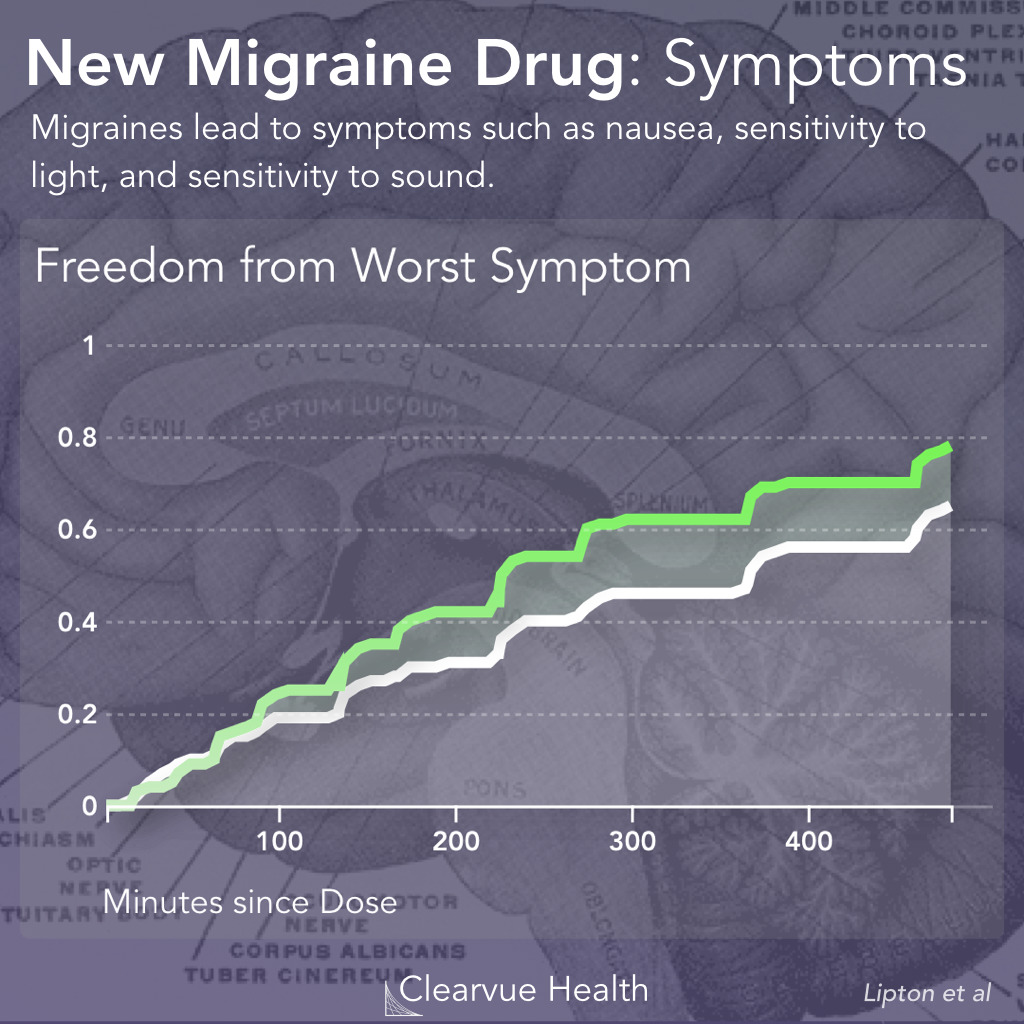
Figure 3: Rimegepant and Migraine Symptoms. Researchers measured how fast patients recovered from their most bothersome symptom other than pain. This symptom was either light sensitivity, sound sensitivity, or nausea. Patients on Rimegepant were significantly more likely to be relieved of their most bothersome symptom other than pain after 2 hours. (37.6% vs 25.2%, p<0.001)
Unlike other headaches, migraines usually carry other symptoms which are very bothersome for patients.
The symptoms are slightly different for each patient. In order to see how effective this drug is at relieving all symptoms, researchers measured how quickly the drug relieved the worst symptom other than pain for each patient, as reported by the patients themselves.
In a similar story to the pain data reported earlier, Rimegepant sped up recovery but it didn’t stop the symptoms. They were also quite a few patients who didn’t necessarily benefit.
Source: Rimegepant, an Oral Calcitonin Gene–Related Peptide Receptor Antagonist, for Migraine
Effect of Rimegepant on Photophobia & Phonophobia
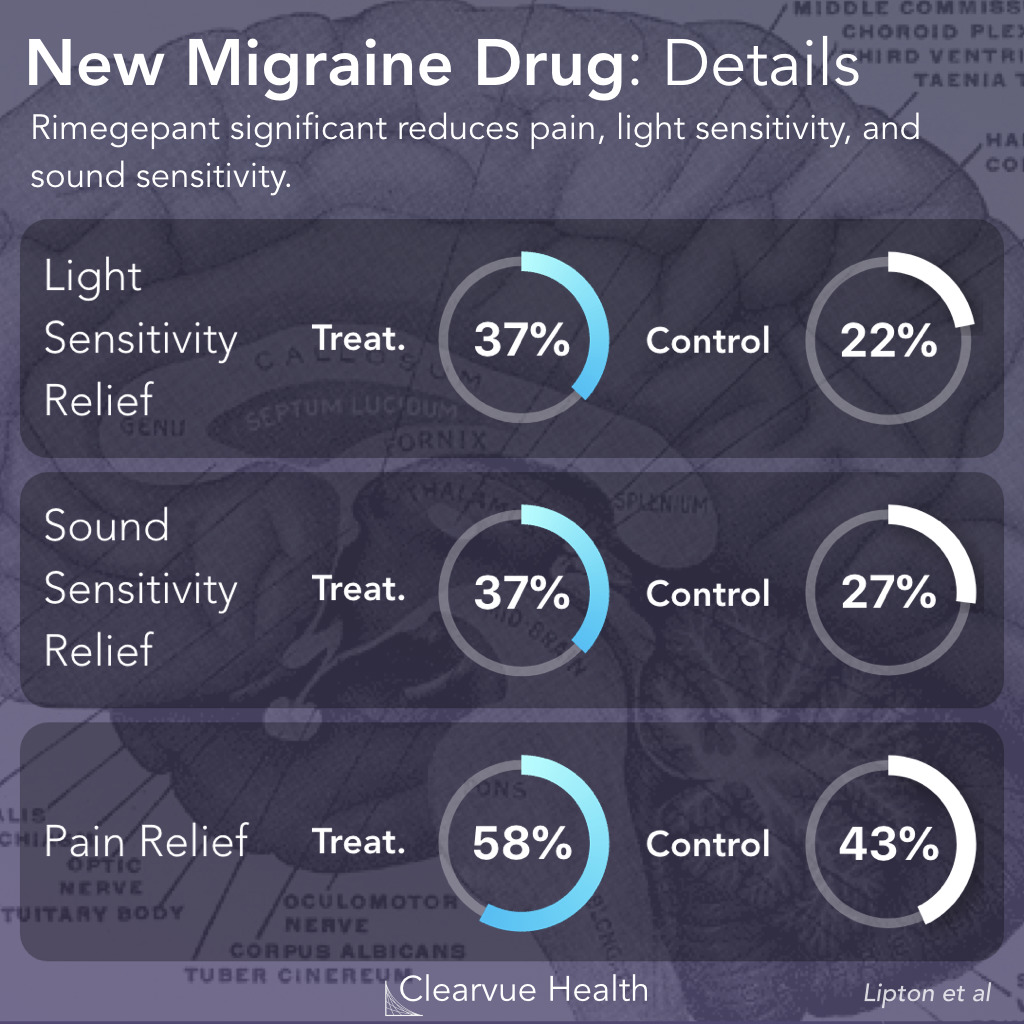
Figure 4: Effect of Rimegepant on Photophobia & Phonophobia. Rimegepant significantly reduced sensitivity to light (photophobia), sensitivity to sound (phonophobia), and pain within the first two hours of a migraine.
Researchers measured symptom relief at 2 hours as a secondary endpoint. They found that sensitivity to light, sensitivity to sound, and pain, were the symptoms that were most likely to be alleviated by Rimegepant.
Nausea, on the other hand, was not significantly alleviated by Rimegepant.
The placebo effect is a strange but powerful effect that affects medicine and clinical trials. When someone believes that a drug will be effective, research has shown over and over again that patients will actually experience relief, whether the drug works or not. If a patient is told that a pill containing nothing more than sugar will make them feel better, patients will typically feel better. This is relevant for clinical trials as scientists must use a placebo control in order to make sure that the results they are seeing are not only due to the placebo effect.
Keys to Health
This study shows that this new drug may be effective in helping to treat migraines.
Unfortunately, this drug does not appear to be a silver bullet in stopping migraines. It only helps a minority of patients, and it only slightly speeds up recovery.
It will however be a useful tool for patients. With the immense pain of a migraine, any drug that can help would be much appreciated by many. Additionally, the results above measure the difference between the drug and a placebo control.
Because of the placebo control comparison, the difference in pain relief will likely be larger between this drug and not taking any drug at all. Placebo controls do tend to reduce symptoms despite not having any active ingredients.
+
Study Type - Researchers used a randomized controlled clinical trial, which is the gold standard for evaluating the effectiveness of a drug.
+
Endpoints - Researchers used freedom from pain and freedom from the most bothersome symptom as the endpoints, which are appropriate symptoms for a migraine drug.
+
Study Size - The study size was large enough to find significant effects and differences between groups.
NEJM
Serotonin 5-HT1B and 5-HT1D receptor agonists (triptans) have been the most widely prescribed acute migraine treatments for decades. However, some patients who use triptans either do not have a response (34%) or have recurrences of attacks (30 to 40%); in addition, up to 52% have adverse effects from triptans, and concerns about these effects were reported in one study to result in delays in treatment or avoidance of treatment in two thirds of patients.
Clearvue Health is not affiliated with above organizations. The information above is provided to highlight and link to useful further reading.