Ritalin Paper Database
Visual Abstract
A double-blind, placebo-controlled study of modified-release methylphenidate in children with attention-deficit/hyperactivity disorder
Study on Modified-Release Methylphenidate in ADHD
September 9, 2024
author
Greenhill LL, Findling RL, Swanson JM
journal
Pediatrics
Date Published
2002 Mar
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
The study focused on evaluating the effectiveness, safety, and ease of use of a once-daily modified-release methylphenidate compared to a placebo in children with ADHD.
💡
What They Found
Children who received methylphenidate showed a significant improvement in ADHD symptoms compared to those who received a placebo. Common side effects in the methylphenidate group included headache, anorexia, abdominal pain, and insomnia, with anorexia being notably more frequent than in the placebo group.
📚
What This Means
The findings suggest that once-daily methylphenidate is an effective treatment for managing ADHD symptoms throughout the school day. This aligns with current evidence which supports methylphenidate as a primary treatment for ADHD in children and adults.
Study Summary
Background
Abstract: background
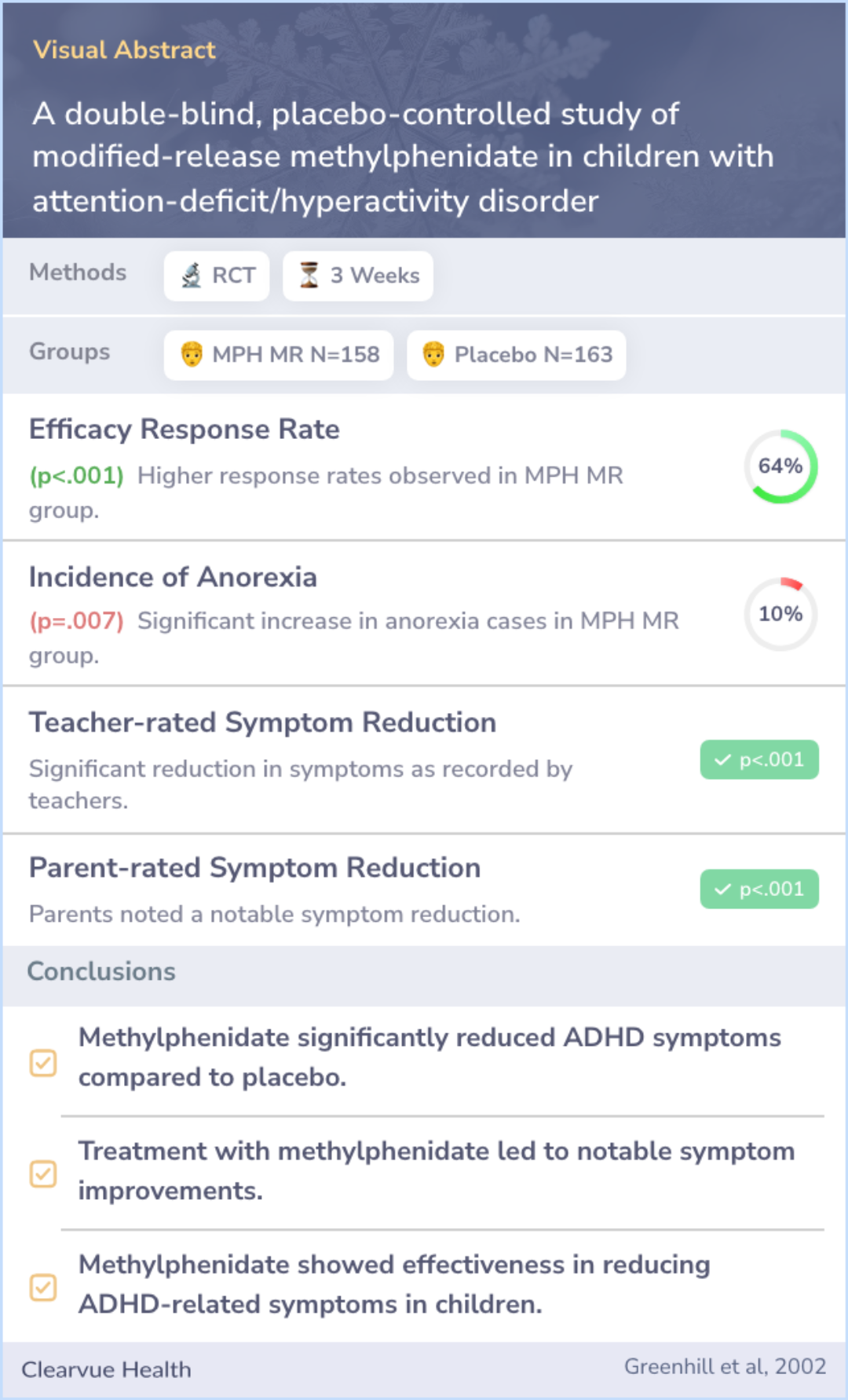
To compare the efficacy, safety, and tolerability of once-daily administration of modified-release methylphenidate (MPH MR) with placebo in children with attention-deficit/hyperactivity disorder (ADHD).
👨⚕️
Methylphenidate's FDA Approval
Methylphenidate is FDA-approved for treating ADHD in children and adults.
🧠
Mechanism of Action
Blocks the reuptake of norepinephrine and dopamine, increasing their concentration in the synaptic cleft.
🚨
Monitoring for Abuse Potential
Due to its Schedule II classification, monitoring for signs of abuse and dependence is critical.
Study Summary
Methods
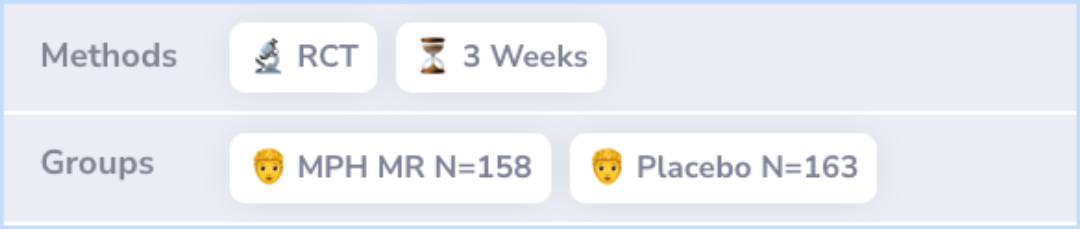
Researchers conducted a 3-week study where they compared MPH MR to a placebo in 32 locations. The participants were children aged 6 to 16 who had ADHD but had not previously undergone unsuccessful treatment with stimulant medications. After a week of taking a placebo to clear any previous medications out of their system, the children were given MPH MR or a placebo daily. The starting dose was one capsule (20 mg), which could be increased up to three capsules (60 mg) based on individual needs. To judge the medication's effectiveness and safety, teachers, parents, and researchers observed changes in ADHD symptoms and noted any side effects.
Abstract: methods
The study was a 3-week, double-blind, 32-site, randomized clinical trial comparing MPH MR with placebo. Children were 6 to 16 years of age, had a diagnosis of ADHD, and had not failed a previous trial of stimulant treatment for ADHD. After a 1-week, ...more
Study Summary
Results
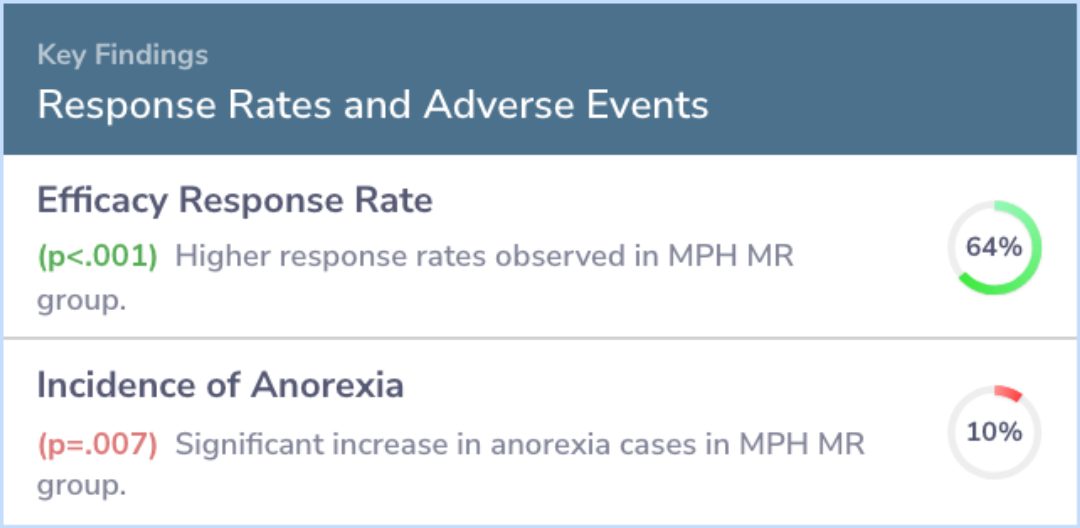
The study included 321 children, splitting them into two groups: 158 received MPH MR, and 163 were given a placebo. Those treated with MPH MR started with a dose of 20 mg per day, which was adjusted to an average of 40.7 mg per day. This group showed significant improvement in ADHD symptoms according to teachers, parents, and researchers compared to the placebo group. Common side effects for the MPH MR group included headaches, lack of appetite, stomach pain, and trouble sleeping, with only loss of appetite being notably more common than in the placebo group.
Abstract: results
The study randomized 321 children: 158 to MPH MR and 163 to placebo. Children in the MPH MR group were started on a dose of 20 mg/d and reached a mean dose of 40.7 mg/d (1.28 mg/kg/d) at endpoint. Compared with placebo, MPH MR significantly reduced A...more

Study Summary
Conclusions
Taking MPH MR once each morning was found to be an effective and safe way to manage symptoms of ADHD in children throughout the school day. Despite some side effects, such as a decreased appetite, the benefits in reducing ADHD symptoms were clearly observed.
Abstract: conclusions
MPH MR administered once daily in the morning is effective and safe in controlling ADHD symptoms throughout the school day.
Professional Guide
Expert Opinion: Study on Modified-Release Methylphenidate in ADHD
The findings suggest that modified-release methylphenidate (MPH MR) significantly reduces ADHD symptoms in children. This is consistent with the meta-analysis showing improvements in ADHD symptoms with extended-release methylphenidate.
Additionally, stimulant medications, including MPH, provide immediate clinical effects, making them beneficial for short-term management.
Current professional recommendations highlight the use of a combination of medication and cognitive-behavioral therapy (CBT) for treating ADHD, addressing both symptoms and executive dysfunction.
Moreover, stimulant medications have common side effects such as insomnia and headaches, which align with the adverse events reported in the study.
Additionally, stimulant medications, including MPH, provide immediate clinical effects, making them beneficial for short-term management.
Current professional recommendations highlight the use of a combination of medication and cognitive-behavioral therapy (CBT) for treating ADHD, addressing both symptoms and executive dysfunction.
Moreover, stimulant medications have common side effects such as insomnia and headaches, which align with the adverse events reported in the study.
Evidence Summary
Efficacy of Combining Methylphenidate with Psychosocial Interventions in ADHD
Building upon efficacy evaluation from the paper above, another study reviewed the impact of combining methylphenidate with intensive multimodal psychosocial intervention, including social skills training.
Children aged 7-9 were treated for two years and assessed for social functioning using various measures.
Results showed no added benefit of the combined treatment over methylphenidate alone or with nonspecific psychosocial treatment.
Social skills training did not show long-term improvements, while methylphenidate benefits remained significant over two years.
Children aged 7-9 were treated for two years and assessed for social functioning using various measures.
Results showed no added benefit of the combined treatment over methylphenidate alone or with nonspecific psychosocial treatment.
Social skills training did not show long-term improvements, while methylphenidate benefits remained significant over two years.
Evidence Summary
Impact of MPH-OROS on OTMP Skills in ADHD
Building upon the efficacy of modified-release methylphenidate (MPH MR) observed in the main study,
this slide examines the impact of another stimulant medication, MPH-OROS, on organizational, time management, and planning (OTMP) skills.
In a double-blind, placebo-controlled trial involving 19 children with ADHD, MPH-OROS significantly improved OTMP behaviors.
Despite these improvements, 61% of children still exhibited OTMP impairments, indicating the need for additional interventions tailored to OTMP deficits.
this slide examines the impact of another stimulant medication, MPH-OROS, on organizational, time management, and planning (OTMP) skills.
In a double-blind, placebo-controlled trial involving 19 children with ADHD, MPH-OROS significantly improved OTMP behaviors.
Despite these improvements, 61% of children still exhibited OTMP impairments, indicating the need for additional interventions tailored to OTMP deficits.
Evidence Summary
Impact of Methylphenidate on Time-Production Task Performance in ADHD
Building upon the exploration of methylphenidate's overall efficacy, the study explored its impact on a specific task.
Children with ADHD participated in a time-production task both with and without methylphenidate.
While the medication didn't alter the number of correct responses or lever hold durations, it did reduce the variability in timing responses.
Notably, it increased the duration of certain lever holds and decreased extremely short lever holds.
Children with ADHD participated in a time-production task both with and without methylphenidate.
While the medication didn't alter the number of correct responses or lever hold durations, it did reduce the variability in timing responses.
Notably, it increased the duration of certain lever holds and decreased extremely short lever holds.