Strattera Paper Database
Visual Abstract
A randomized double-blind study of atomoxetine versus placebo for attention-deficit/hyperactivity disorder symptoms in children with autism spectrum disorder
Study of Atomoxetine vs Placebo for ADHD in ASD
September 14, 2024
author
Harfterkamp M, van de Loo-Neus G, Minderaa RB, van der Gaag RJ, Escobar R, Schacht A, Pamulapati S, Buitelaar JK, Hoekstra PJ
journal
J Am Acad Child Adolesc Psychiatry
Date Published
2012 Jul
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
The study investigated whether atomoxetine is effective for treating ADHD symptoms in patients with autism spectrum disorder.
💡
What They Found
The study found that atomoxetine moderately improved ADHD symptoms in patients with ASD compared to placebo, without causing serious adverse events.
📚
What This Means
These findings suggest that atomoxetine may offer moderate benefits for ADHD symptoms in children with ASD, aligning with existing evidence on ADHD medication efficacy.
Study Summary
Study Overview
Researchers aimed to explore if atomoxetine, a medication for ADHD, could help children with autism spectrum disorder (ASD). They hoped to see if it worked better than a placebo after eight weeks of treatment. They found that while atomoxetine positively affected hyperactivity more than inattention, its overall impact was less pronounced in children with ASD compared to those with typical ADHD. This points to the complexities of treating ADHD in children with ASD.
Overall, the study sheds light on atomoxetine's effects, suggesting it could offer some benefits for managing ADHD symptoms in children with ASD, even as challenges remain.
Overall, the study sheds light on atomoxetine's effects, suggesting it could offer some benefits for managing ADHD symptoms in children with ASD, even as challenges remain.
Abstract: background
The efficacy of atomoxetine as treatment of symptoms of attention-deficit/hyperactivity disorder (ADHD) in patients with autism spectrum disorder (ASD) has not been established.

Study Overview
"This is the first adequately powered placebo-controlled study examining the efficacy and safety of atomoxetine for symptoms of ADHD in children with ASD."
Treatment Challenges
"The relatively lesser effect of atomoxetine in children with ASD compared to children with typical ADHD highlights the challenge of treating ADHD symptoms in children with ASD, where comorbid conditions complicate the clinical picture."
Study Findings
"Although the current version of the DSM precludes a diagnosis of ADHD in patients with ASD, a wealth of recent clinical and population-based studies have pointed to the relevance of ADHD in ASD."
Study Summary
Methods
Researchers conducted an experiment involving 97 participants, aged 6 to 17 years, who had both ADHD and ASD. These participants were randomly divided into two groups. One group received atomoxetine at a dosage of 1.2 mg/kg/day while the other was given a placebo, a pill containing no medicine, for a duration of 8 weeks.
The primary measure of success was changes in the ADHD Rating Scale (ADHD-RS). Secondary measures included scores from the Clinical Global Impression of ADHD-Improvement (CGI-I) and the Conners Teacher Rating Scale-Revised: Short Form (CTRS-R:S).
The primary measure of success was changes in the ADHD Rating Scale (ADHD-RS). Secondary measures included scores from the Clinical Global Impression of ADHD-Improvement (CGI-I) and the Conners Teacher Rating Scale-Revised: Short Form (CTRS-R:S).
Abstract: methods
In this study, 97 patients aged 6 to 17 years with ADHD and ASD were randomly assigned to double-blind treatment with 1.2 mg/kg/day atomoxetine or placebo for 8 weeks. The primary endpoint was the ADHD Rating Scale (ADHD-RS) score; secondary endpoint...more
Study Summary
Results
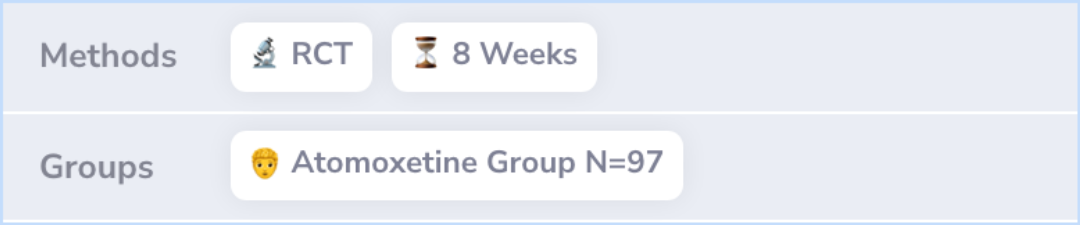
Results showed that after 8 weeks, those taking atomoxetine had noticeable improvement in their ADHD-RS scores compared to those who received a placebo.
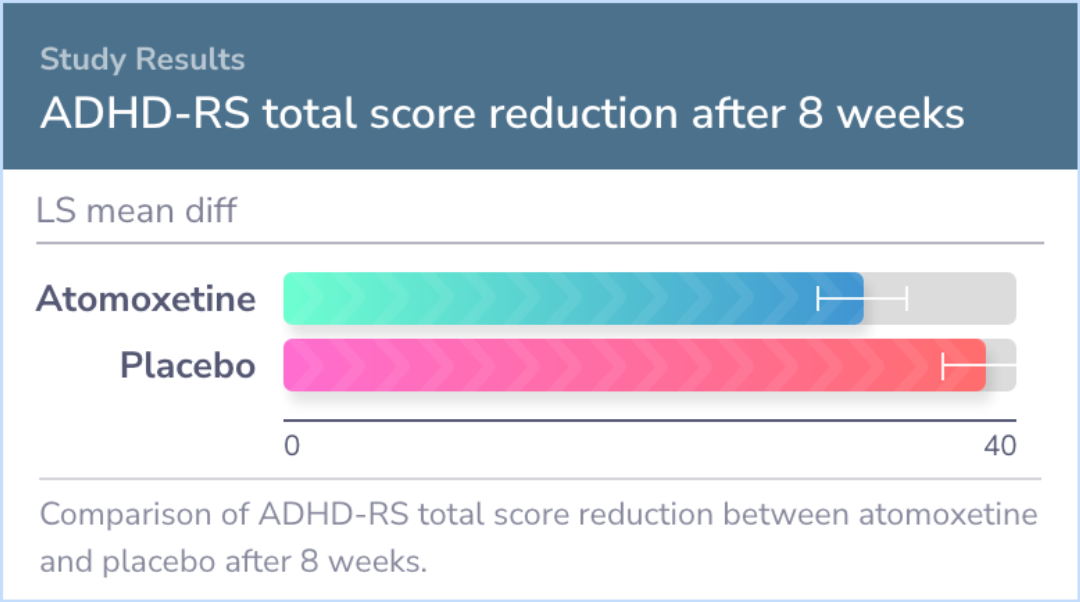
The hyperactivity part of the CTRS-R:S score also improved for those on atomoxetine. However, according to the CGI-I scores, there wasn't a significant difference in improvement levels between the atomoxetine and placebo groups. Participants did experience some side effects, such as nausea and fatigue, but these were not serious and are commonly reported in other studies involving ADHD medications.
The hyperactivity part of the CTRS-R:S score also improved for those on atomoxetine. However, according to the CGI-I scores, there wasn't a significant difference in improvement levels between the atomoxetine and placebo groups. Participants did experience some side effects, such as nausea and fatigue, but these were not serious and are commonly reported in other studies involving ADHD medications.
Abstract: results
Baseline mean ADHD-RS scores for atomoxetine versus placebo were 40.7 and 38.6; after 8 weeks, mixed-effect model repeated-measure means were 31.6 (95% confidence interval 29.2-33.9) and 38.3 (36.0-40.6), respectively, with a difference in least squa...more

Study Summary
Conclusions
In summary, atomoxetine was found to moderately improve ADHD symptoms in young patients with ASD and was generally well tolerated. This means the medication's side effects were similar to those observed in individuals with only ADHD.
These results offer a glimpse into potential treatment avenues for managing ADHD symptoms in children with ASD, though more research is needed to fully understand the best approaches.
These results offer a glimpse into potential treatment avenues for managing ADHD symptoms in children with ASD, though more research is needed to fully understand the best approaches.
Abstract: conclusions
Atomoxetine moderately improved ADHD symptoms in patients with ASD and was generally well tolerated. Adverse events in this study were similar to those in other studies with ADHD patients without ASD. Clinical trial registration information-A Randomi...more

Background Information
Patient Guide
⚙️
Norepinephrine Reuptake Inhibition
Atomoxetine selectively inhibits norepinephrine reuptake, influencing ADHD symptoms by affecting dopamine in specific brain regions.
📏
Growth Monitoring in Children
Children on atomoxetine need regular checks for growth, as treatment may affect height and weight over time.
🤕
Common Adverse Effects
Patients frequently experience side effects like nausea, decreased appetite, and insomnia while on atomoxetine.
❤️
Cardiac Contraindications
Atomoxetine is contraindicated in patients with serious cardiac conditions, due to potential cardiovascular risks.
Professional Guide
Expert Opinion: Study of Atomoxetine vs Placebo for ADHD in ASD
The findings show that atomoxetine moderately improved ADHD symptoms in patients with ASD, with tolerable side effects.
Current professional recommendations highlight the importance of monitoring for suicidal ideation, growth, and cardiovascular health in children and adolescents on atomoxetine.
This is consistent with the observed effects of atomoxetine in both ADHD and ASD populations.
Current professional recommendations highlight the importance of monitoring for suicidal ideation, growth, and cardiovascular health in children and adolescents on atomoxetine.
This is consistent with the observed effects of atomoxetine in both ADHD and ASD populations.
Evidence Summary
Boosting ADHD Outcomes: Medication Plus Therapy
Methylphenidate combined with psychosocial treatments tackles ADHD symptoms effectively, enhancing attention and behavior. This combination proves more beneficial than employing either method in isolation. Patients experience improved outcomes through this dual approach.
The abstract explores another route, examining an ADHD medication, atomoxetine, in different contexts, revealing different impacts compared to methylphenidate strategies.
The abstract explores another route, examining an ADHD medication, atomoxetine, in different contexts, revealing different impacts compared to methylphenidate strategies.
Evidence Summary
Balancing Methylphenidate Dosage and Side Effects in ADHD Treatment
Research on methylphenidate for ADHD explores the careful balancing act between dosage and side effects, stressing the value of personalized approaches. Methylphenidate dosages showed varying levels of success in managing ADHD symptoms while impacting side effects differently. Individualized treatment plans, tailored to each child’s needs, emerged as key for optimizing effectiveness and minimizing unwanted effects.
Evidence Summary
Adderall XR: Enhancing Attention and Behavior in ADHD
Exploring the daily use of Adderall XR, this article dives into its role in enhancing attention and behavior in children with ADHD. A study reviews its effectiveness and safety, highlighting how a once-a-day dosage can manage symptoms effectively.
The text ties in with the previous discussion by providing a distinct look into how children with ADHD benefit from Adderall XR, separate from the other treatments mentioned.
The text ties in with the previous discussion by providing a distinct look into how children with ADHD benefit from Adderall XR, separate from the other treatments mentioned.
Evidence Summary
ADHD Subtypes and Medication Response
The discussion centers around how different ADHD subtypes react to methylphenidate. Each subtype of ADHD shows variations in how effective the medication is, showcasing a diverse range of responses. Understanding these reactions is tied to the executive function abilities of the children.
This investigation reveals that ADHD subtypes not only differ in their traits but also in how well they respond to treatment, highlighting a nuanced relationship with executive functions.
This investigation reveals that ADHD subtypes not only differ in their traits but also in how well they respond to treatment, highlighting a nuanced relationship with executive functions.




