Trending ADHD Papers
Visual Abstract
Combined Methylphenidate and Selective Serotonin Reuptake Inhibitors in Adults With Attention-Deficit/Hyperactivity Disorder
Methylphenidate and SSRI Combination in ADHD
December 1, 2024
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
Evaluate the safety of combining SSRI and methylphenidate in adults with ADHD and comorbid depression.
💡
What They Found
The study found no significant increase in adverse events when using SSRI with methylphenidate compared to methylphenidate alone, with the combination showing a lower risk of headaches.
📚
What This Means
The findings suggest that using an SSRI with methylphenidate does not increase the risk of adverse events in adults with ADHD and depression, and may even reduce headache risk, aligning with the current understanding of methylphenidate's applications for ADHD.
Study Overview
Background & Objectives
Methylphenidate, a stimulant for treating ADHD, works by boosting neurotransmitter levels in the brain. Often prescribed to both children and adults, it also serves off-label purposes such as treating geriatric depression.
The study aims to assess the safety of combining methylphenidate with SSRIs in adults who have both ADHD and depression, monitoring various health outcomes and comparing this drug balance with methylphenidate alone.
The study aims to assess the safety of combining methylphenidate with SSRIs in adults who have both ADHD and depression, monitoring various health outcomes and comparing this drug balance with methylphenidate alone.
Abstract: background
To evaluate the safety of administering a combination of SSRI and methylphenidate in adults with ADHD and comorbid depression.
Study Summary
Methods
Researchers used a nationwide database from South Korea, spanning January 2016 to February 2021. The study included adults aged 18 and older with ADHD and depression, all prescribed methylphenidate.
Participants were divided into groups based on their prescribed medications. Advanced statistical methods, like hazard ratios, were used to compare risks, and the groups were balanced to account for differences in age, sex, and other factors.
Participants were divided into groups based on their prescribed medications. Advanced statistical methods, like hazard ratios, were used to compare risks, and the groups were balanced to account for differences in age, sex, and other factors.

Abstract: methods
This cohort study obtained data from a nationwide claims database in South Korea from January 2016 to February 2021. Participants were ...more
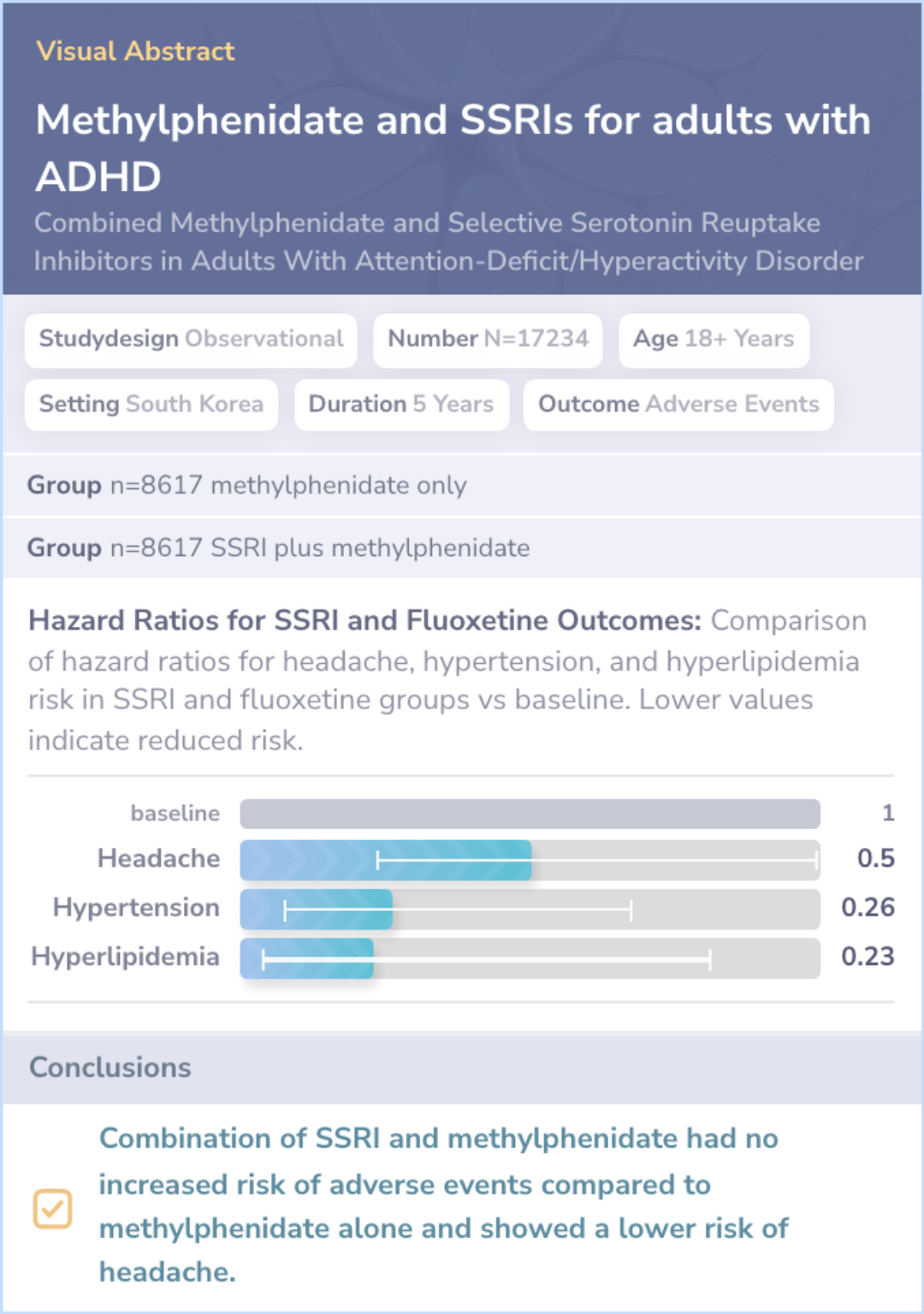
Study Results
Results
The study included over 17,000 adults with ADHD and depression, with an average age of 29 years. No increased risk of adverse events was found when SSRIs were combined with methylphenidate. The SSRI group even showed a reduced risk of headaches.
Comparing SSRIs, fluoxetine users had lower risks of conditions like hypertension and hyperlipidemia than escitalopram users, according to sensitivity analyses.
Comparing SSRIs, fluoxetine users had lower risks of conditions like hypertension and hyperlipidemia than escitalopram users, according to sensitivity analyses.
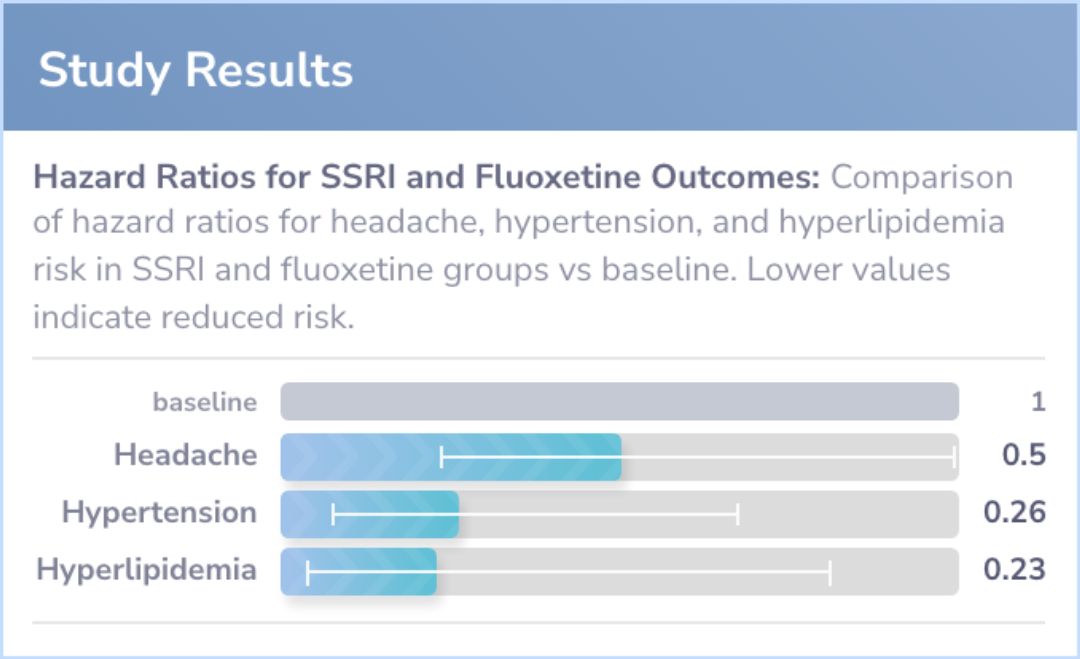
Abstract: results
The study included 17,234 adults with ADHD (mean [SD] age at study entry, 29.4 [10.8] years; 9,079 females [52.7%]). There was no diffe...more
Study Summary
Conclusions
The findings suggest that combining SSRIs with methylphenidate does not increase the risk of adverse events in adults with ADHD and depression. In fact, some potential benefits, such as fewer headaches, were observed.
Among SSRIs, fluoxetine may offer lower risks for certain health conditions compared to escitalopram, indicating it could be a preferable option for some patients.
Among SSRIs, fluoxetine may offer lower risks for certain health conditions compared to escitalopram, indicating it could be a preferable option for some patients.
Abstract: conclusions
Results of this study revealed no significant increase in adverse event risk associated with use of SSRI plus methylphenidate vs methylphenidate alone in adults with ADHD and comorbid depression. Instead, the combination was associated with a lower r...more
Clinical Guidelines
Guidelines suggest that monitoring for serotonin syndrome is important when combining ADHD medications with SSRIs.
Fluoxetine and escitalopram differ in cardiovascular risks when used with stimulants; fluoxetine presents lower hypertension and hyperlipidemia risks.
Stimulant medications are recommended as first-line treatment for adult ADHD.
Fluoxetine and escitalopram differ in cardiovascular risks when used with stimulants; fluoxetine presents lower hypertension and hyperlipidemia risks.
Stimulant medications are recommended as first-line treatment for adult ADHD.
Literature Review
Kim et al., 2023
Core Insight:Both studies find no significant increase in adverse events from combining methylphenidate and selective serotonin reuptake inhibitors (SSRIs) in individuals with ADHD and depression.
What It Adds:
Focus on Age Groups: The comparison paper focuses on adolescents, while the main paper examines adults, expanding the findings across different age groups.
Different Outcomes Assessed: The comparison paper includes tic disorder as an outcome, which was not part of the main paper's study.
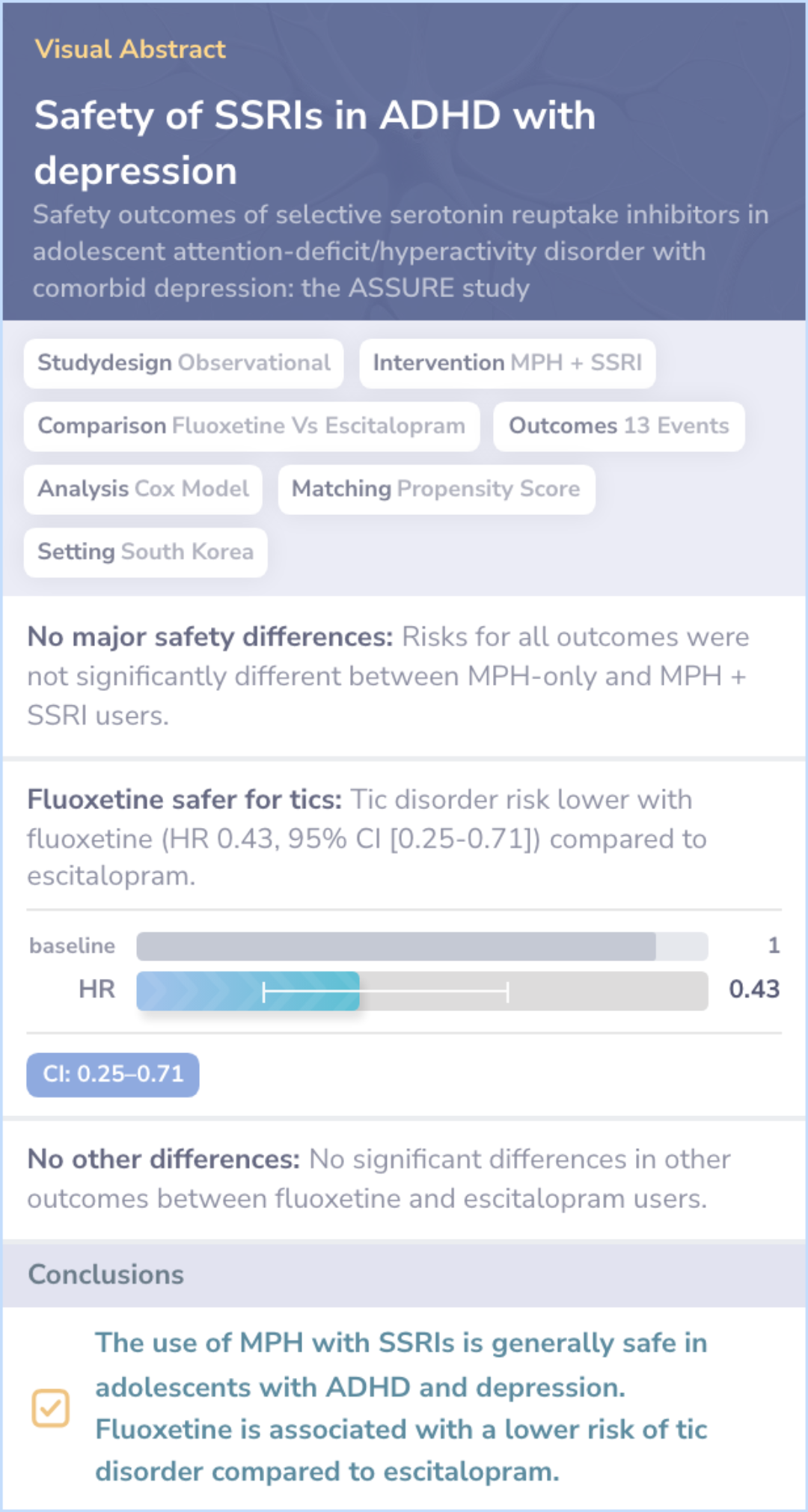
Literature Review
Park et al, 2022
Core Insight:Both studies focus on ADHD treatment, with one addressing the safety of combining methylphenidate with SSRIs in adults and the other examining the long-term risks of methylphenidate in children.
What It Adds:
No added risks with SSRI in adults: The main paper found no increase in adverse events with methylphenidate and SSRIs.
Long-term methylphenidate reduces risks: The comparison paper found reduced risks of depression and conduct issues with long-term use.
Different population focus: The main paper examines adults; the comparison paper focuses on children.
Key Differences:
Population and treatment scope: Main paper examines adults with comorbid depression; comparison paper studies children with long-term methylphenidate use.
Outcomes investigated: The main paper evaluates SSRIs' safety; the comparison paper assesses long-term risks of methylphenidate.
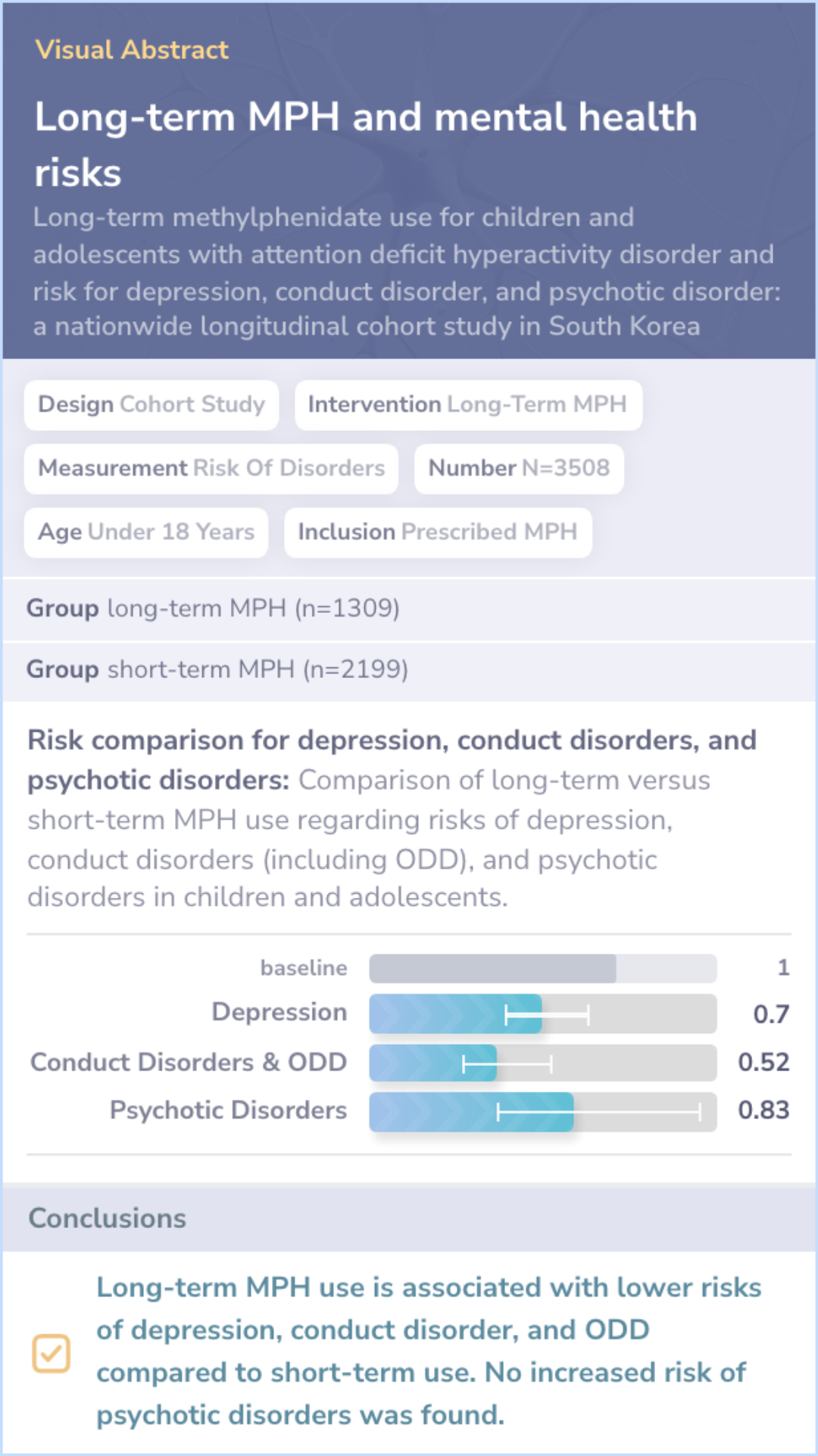
Literature Review
Adler et al, 2006
Core Insight:The main paper evaluates the safety of combining SSRIs and methylphenidate in adults with ADHD and depression, finding no significant adverse risks, while the comparison paper highlights the broader use of combination therapies for associated conditions and side effects, like insomnia.
What It Adds:
Combination therapies validated: The comparison paper supports using combination treatments for partial responses or side effects.
Specific benefits noted: Adding mirtazapine reduced insomnia, while additional stimulants extended therapeutic duration.
Key Differences:
Focus on specific combinations: The main paper centers on methylphenidate and SSRIs, unlike the broader scope of the comparison paper.
Outcomes explored: The main paper assesses safety and adverse events, while the comparison paper highlights effectiveness and side effect management.
