Strattera Paper Database
Visual Abstract
Long-term atomoxetine treatment in adolescents with attention-deficit/hyperactivity disorder
Long-term atomoxetine treatment in adolescents with ADHD
September 14, 2024
author
Wilens TE, Newcorn JH, Kratochvil CJ, Gao H, Thomason CK, Rogers AK, Feldman PD, Levine LR
journal
J Pediatr
Date Published
2006 Jul
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
The main research question was to determine the efficacy and safety of atomoxetine in adolescents treated for ADHD over a period of up to two years.
💡
What They Found
The study found that atomoxetine maintained its efficacy over two years in adolescents with ADHD, with no evidence of drug tolerance and no new safety concerns.
📚
What This Means
The findings align with current evidence indicating that atomoxetine is an effective treatment for ADHD in adolescents, with no unexpected safety issues arising from long-term use.
Study Summary
Study Overview
This study aimed to see how effective atomoxetine is for treating ADHD in adolescents over a long period. It found that the improvement in symptoms mostly happens in the first months but continues steadily. The researchers noted that while some growth rate impacts might occur initially, they don't seem to last long. The study's results provide reassurance for continued treatment without major new safety concerns.
They also found no need to change doses significantly, suggesting atomoxetine remains effective without much risk of tolerance.
They also found no need to change doses significantly, suggesting atomoxetine remains effective without much risk of tolerance.
Abstract: background
To determine the efficacy and safety of atomoxetine in adolescent subjects treated for attention-deficit/hyperactivity disorder (ADHD) for up to 2 years.

Sustained Efficacy
"The current findings of sustained efficacy in the treatment of ADHD are similar to those in prepubertal children receiving stimulants, atomoxetine, or other non-stimulants, as well as in adults receiving stimulants or atomoxetine, indicating continued effectiveness of the ADHD medications accompanied by a low rate of discontinuation due to lack of effectiveness."
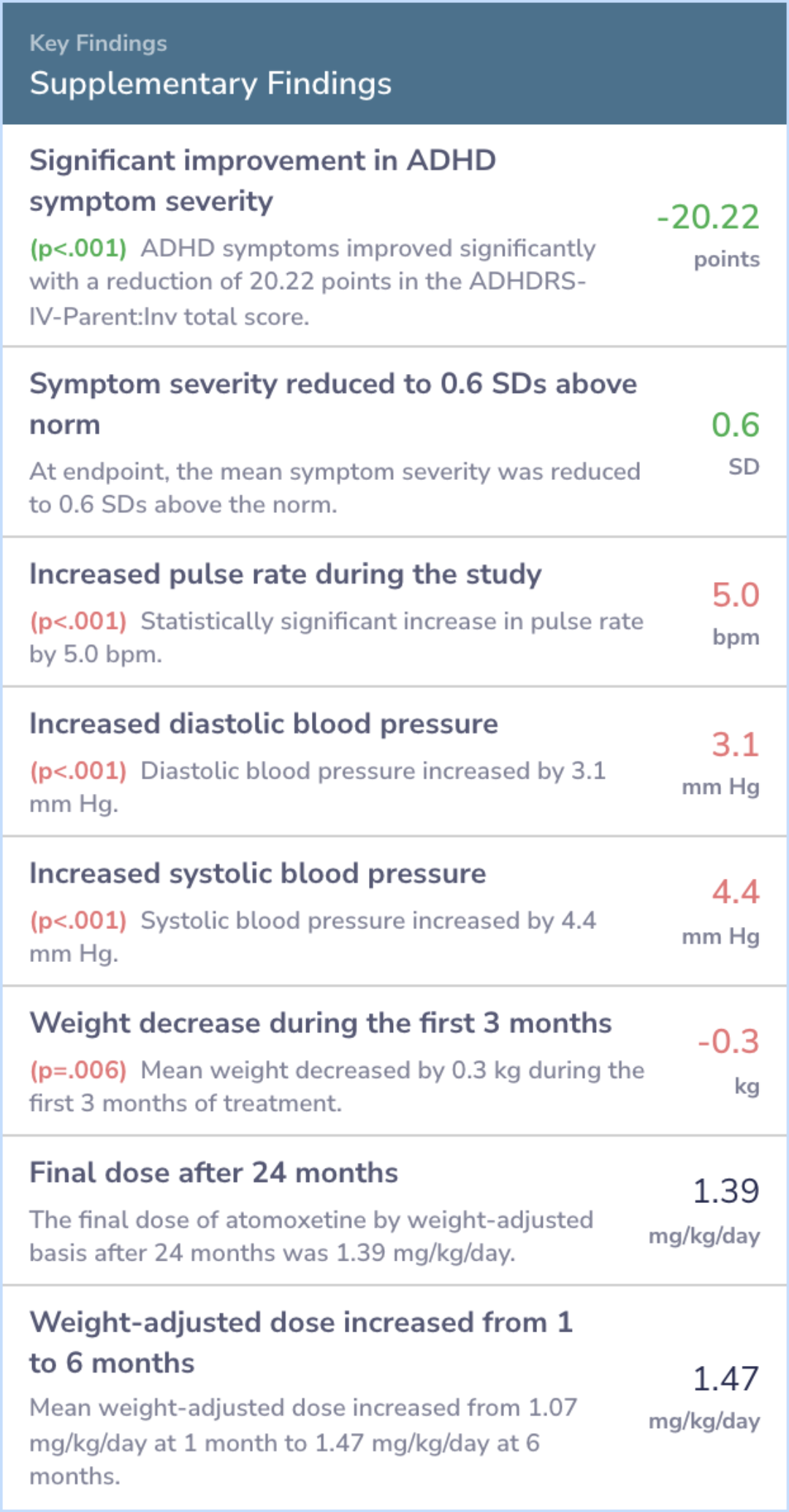
Symptom Improvement
"Most of the symptom improvement occurred within the first 3 months of treatment. Thereafter, response to treatment was maintained over the ensuing period, out to 24 months after the initiation of treatment."
Long-term Effects
"Despite lingering controversy over height and weight growth rate deficits in younger children treated with stimulants, there was no indication that any significant height growth rate deficits seen in these adolescents treated for up to 2 years would be long-lasting."
Study Summary
Methods
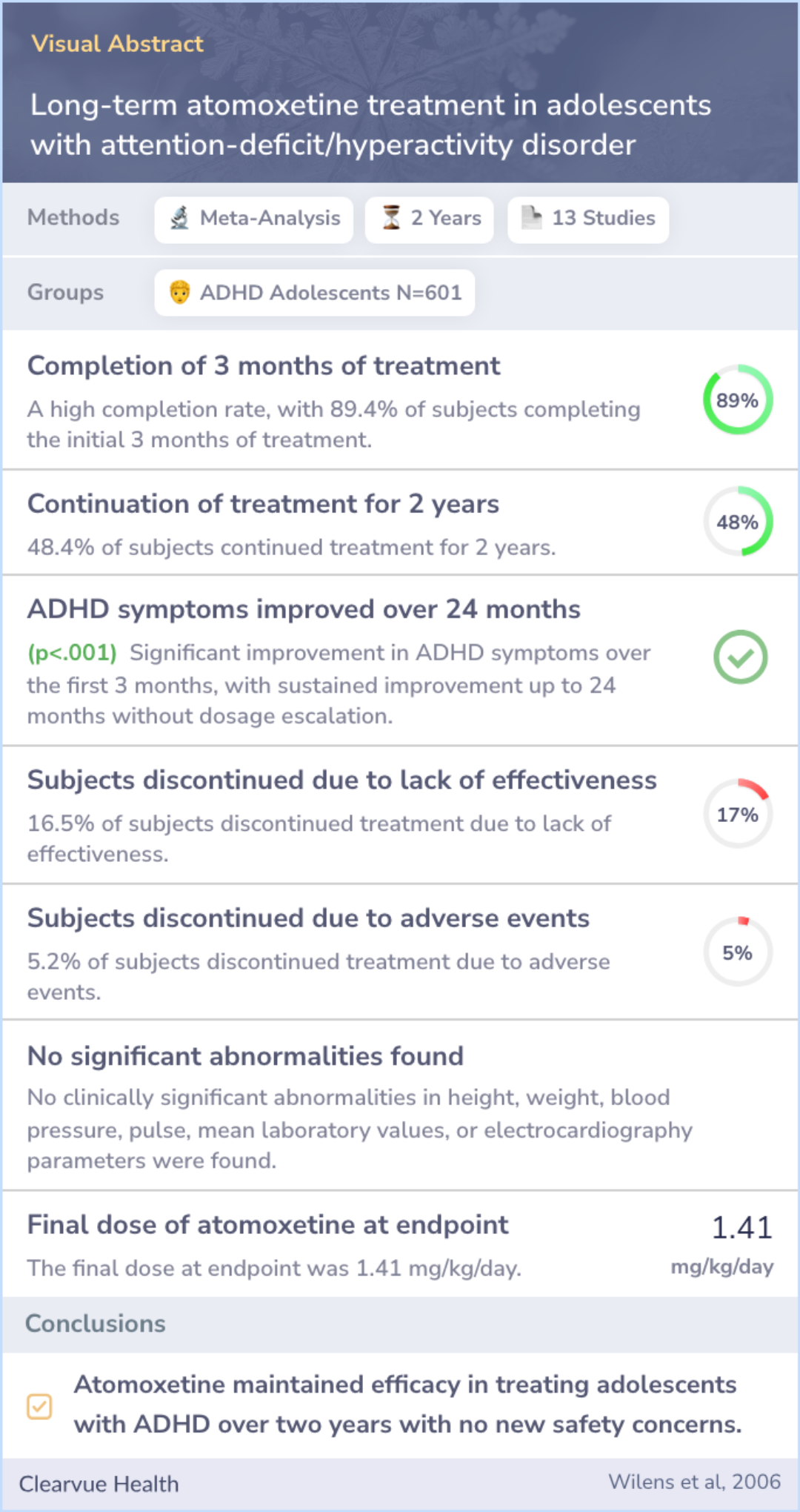
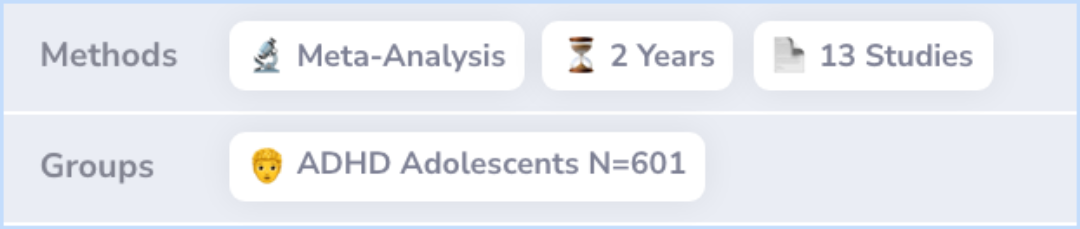
Researchers gathered data from 13 studies involving atomoxetine. These included both placebo-controlled and open-label studies, which means some were blinded so participants didn't know if they were receiving the drug or a placebo.
The participants involved were adolescents between the ages of 12 to 18, diagnosed with ADHD based on established criteria. This approach helps ensure the findings are robust and applicable to a wide adolescent population.
The participants involved were adolescents between the ages of 12 to 18, diagnosed with ADHD based on established criteria. This approach helps ensure the findings are robust and applicable to a wide adolescent population.
Abstract: methods
Data from 13 atomoxetine studies (6 double-blind, 7 open-label) were pooled for subjects age 12 to 18 with ADHD as defined by the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders IV.
Study Summary
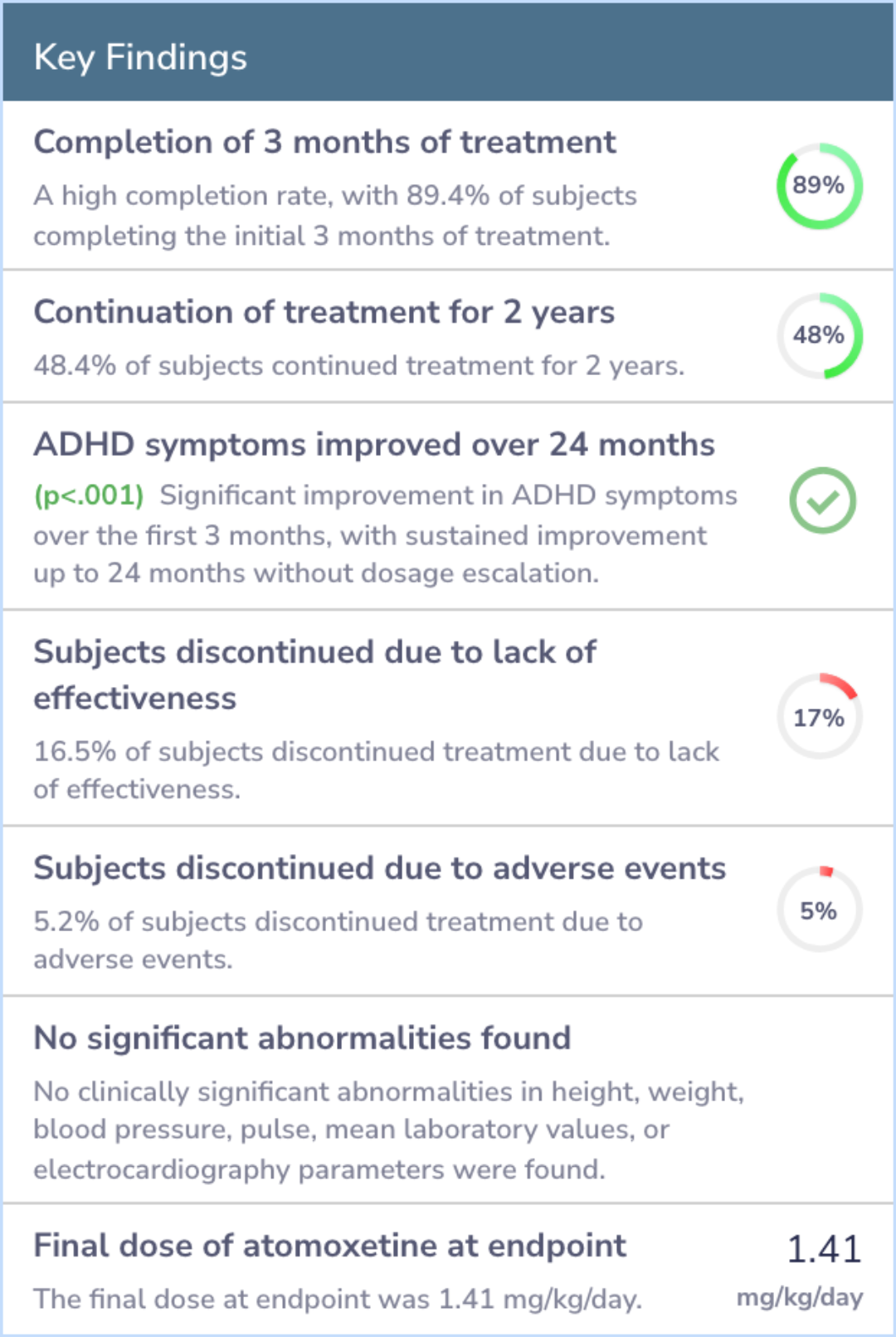
Results
In this analysis, most participants (almost 90%) completed three months of treatment with atomoxetine, and nearly half continued treatment for up to two years. Notably, those who remained on the medication showed significant improvement within the first three months.
Moreover, the benefits observed were sustained over two years without increasing doses. Side effects were minimal, with few discontinuing due to adverse events, and there were no significant changes in vital health metrics like height or blood pressure.
Moreover, the benefits observed were sustained over two years without increasing doses. Side effects were minimal, with few discontinuing due to adverse events, and there were no significant changes in vital health metrics like height or blood pressure.
Abstract: results
Of the 601 atomoxetine-treated subjects in this meta-analysis, 537 (89.4%) completed 3 months of acute treatment. A total of 259 subjects (48.4%) are continuing atomoxetine treatment; 219 of these subjects have completed at least 2 years of treatment...more
Study Summary
Conclusions
The study concluded that atomoxetine is effective in maintaining symptom control in adolescents with ADHD over a prolonged period. Importantly, there is no indication that the medication loses effectiveness over time or that patients develop tolerance.
No new safety issues emerged during the study, reinforcing atomoxetine’s suitability as a long-term treatment option for teenagers dealing with ADHD.
No new safety issues emerged during the study, reinforcing atomoxetine’s suitability as a long-term treatment option for teenagers dealing with ADHD.
Abstract: conclusions
Two-year data from this ongoing study indicate that atomoxetine maintains efficacy among adolescents with ADHD, with no evidence of drug tolerance and no new or unexpected safety concerns.

Background Information
Patient Guide
💊
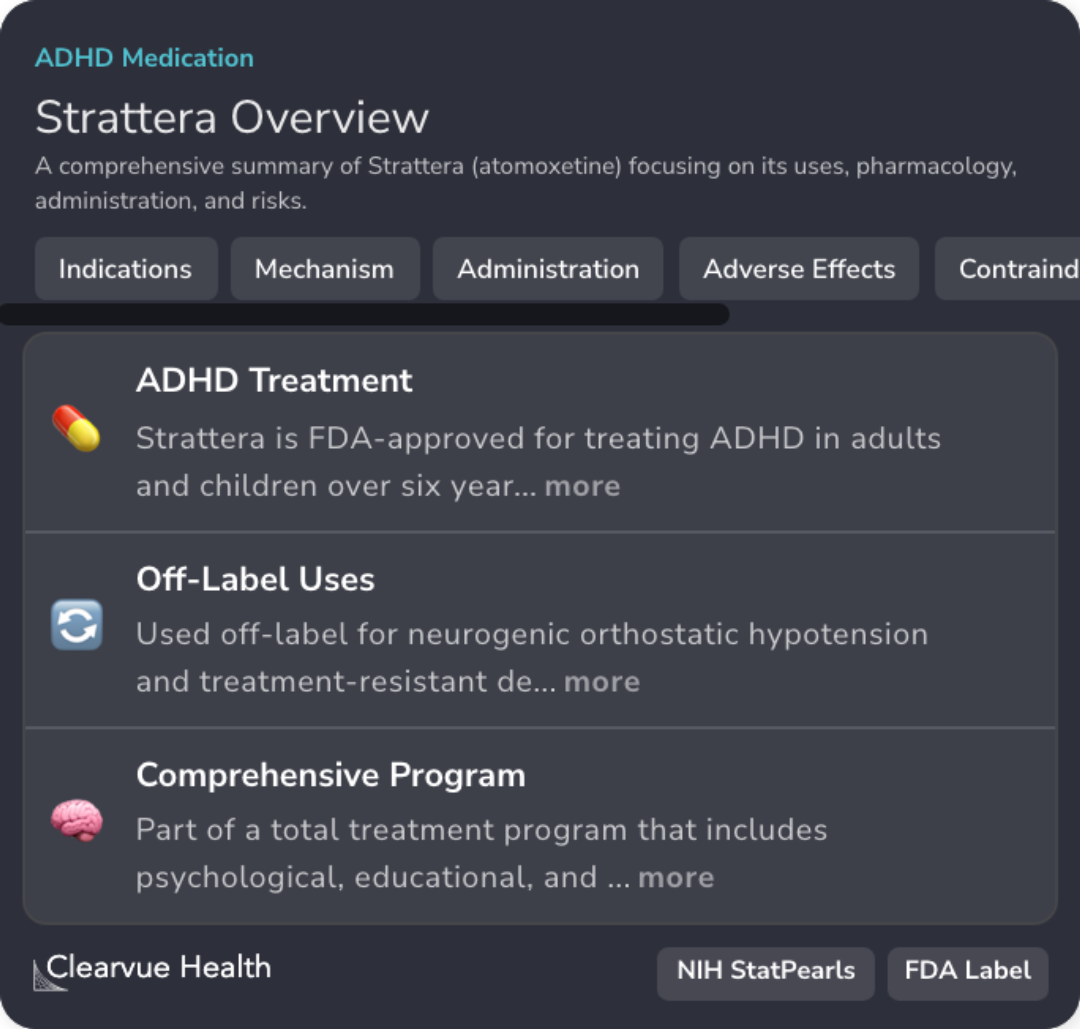
FDA Approval for ADHD
Strattera is FDA-approved for treating ADHD in adolescents over six years old.
⚙️
Mechanism as Norepinephrine Inhibitor
Atomoxetine acts as a selective norepinephrine reuptake inhibitor, affecting dopamine in specific brain areas.
🤕
Potential Adverse Effects
Common adverse effects include headaches, insomnia, and appetite changes, requiring careful monitoring.
🧠
Psychiatric Monitoring
Patients on atomoxetine need monitoring for psychiatric symptoms, as it carries a risk of suicidal ideation.
📏
Importance of Growth Monitoring
Regular checks of height and weight are advised for children on atomoxetine due to possible growth effects.
Professional Guide
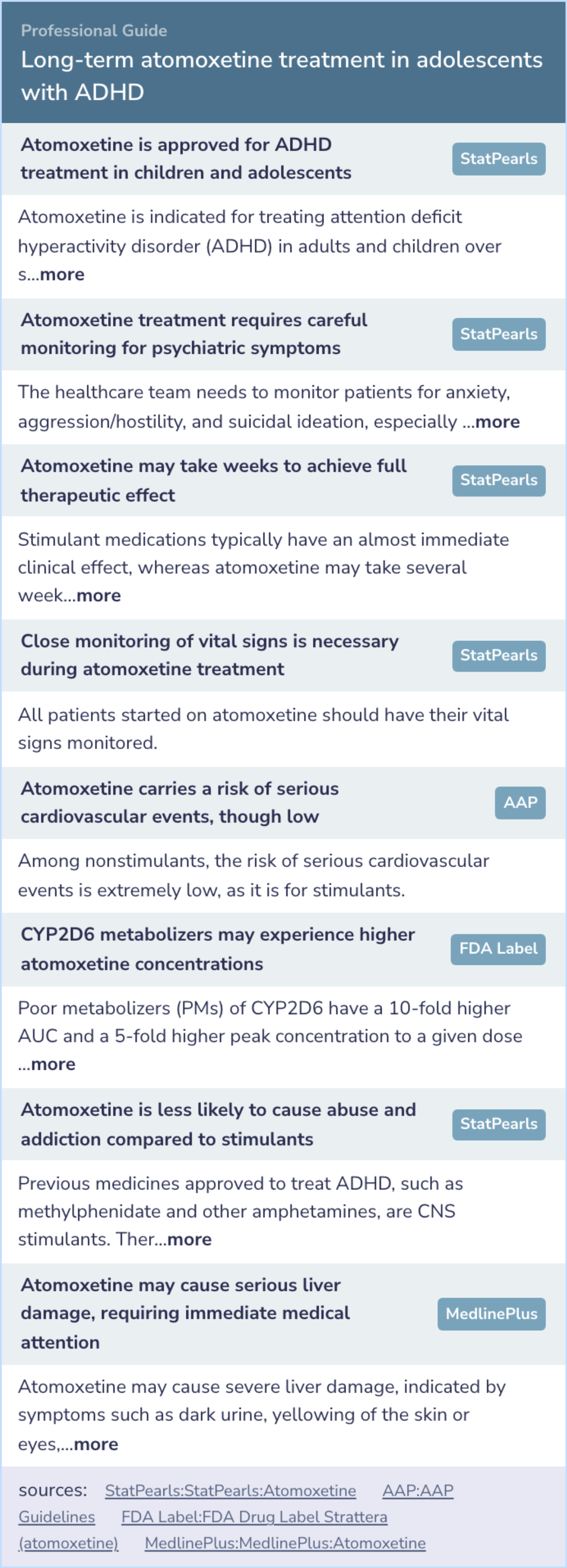
Expert Opinion: Long-term atomoxetine treatment in adolescents with ADHD
Atomoxetine's role in ADHD treatment for adolescents correlates with findings, underscoring its approval for use in this group.
Healthcare professionals must carefully monitor patients for psychiatric symptoms during treatment, ensuring patient safety.
Unlike stimulants, atomoxetine requires time to reveal its therapeutic effects, highlighting the need for patience in treatment.
Vital sign monitoring is integral in atomoxetine therapy to avoid potential cardiovascular concerns.
Moreover, lower abuse potential makes atomoxetine a safer option compared to traditional stimulants.
Healthcare professionals must carefully monitor patients for psychiatric symptoms during treatment, ensuring patient safety.
Unlike stimulants, atomoxetine requires time to reveal its therapeutic effects, highlighting the need for patience in treatment.
Vital sign monitoring is integral in atomoxetine therapy to avoid potential cardiovascular concerns.
Moreover, lower abuse potential makes atomoxetine a safer option compared to traditional stimulants.
Evidence Summary
Comparing Atomoxetine and Methylphenidate for ADHD Treatment
Atomoxetine and Methylphenidate, both prescribed for ADHD, are examined side by side to reveal their strengths, side effects, and patient reactions. The comparison gives a clear view of how these medications differ in their impact on patients.
By exploring the effectiveness and side effects of each, readers gain a better sense of what to expect from these treatments and can weigh the options based on the experiences of others.
By exploring the effectiveness and side effects of each, readers gain a better sense of what to expect from these treatments and can weigh the options based on the experiences of others.
Evidence Summary
Comparing the Effectiveness of Methylphenidate and Atomoxetine
Methylphenidate and atomoxetine are two medications used to manage ADHD symptoms. Each drug offers different benefits depending on the individual's needs, and the comparison highlights their effectiveness. This discussion provides insights into how these medications work and what distinguishes one from the other.
The comparison helps clarify which medication might be more suitable for certain cases of ADHD, emphasizing their roles and differences in treatment.
The comparison helps clarify which medication might be more suitable for certain cases of ADHD, emphasizing their roles and differences in treatment.
Evidence Summary
Combining Atomoxetine and Parental Support for ASD and ADHD
Atomoxetine shows promise in helping children with both Autism Spectrum Disorder (ASD) and ADHD manage their symptoms. Alongside medication, parent training programs can be an effective tandem approach, suggesting that support from family plays a crucial role. Researchers are investigating the synergistic effects of combining these treatments to enhance outcomes for children facing the dual challenges of ASD and ADHD.