Strattera Paper Database
Visual Abstract
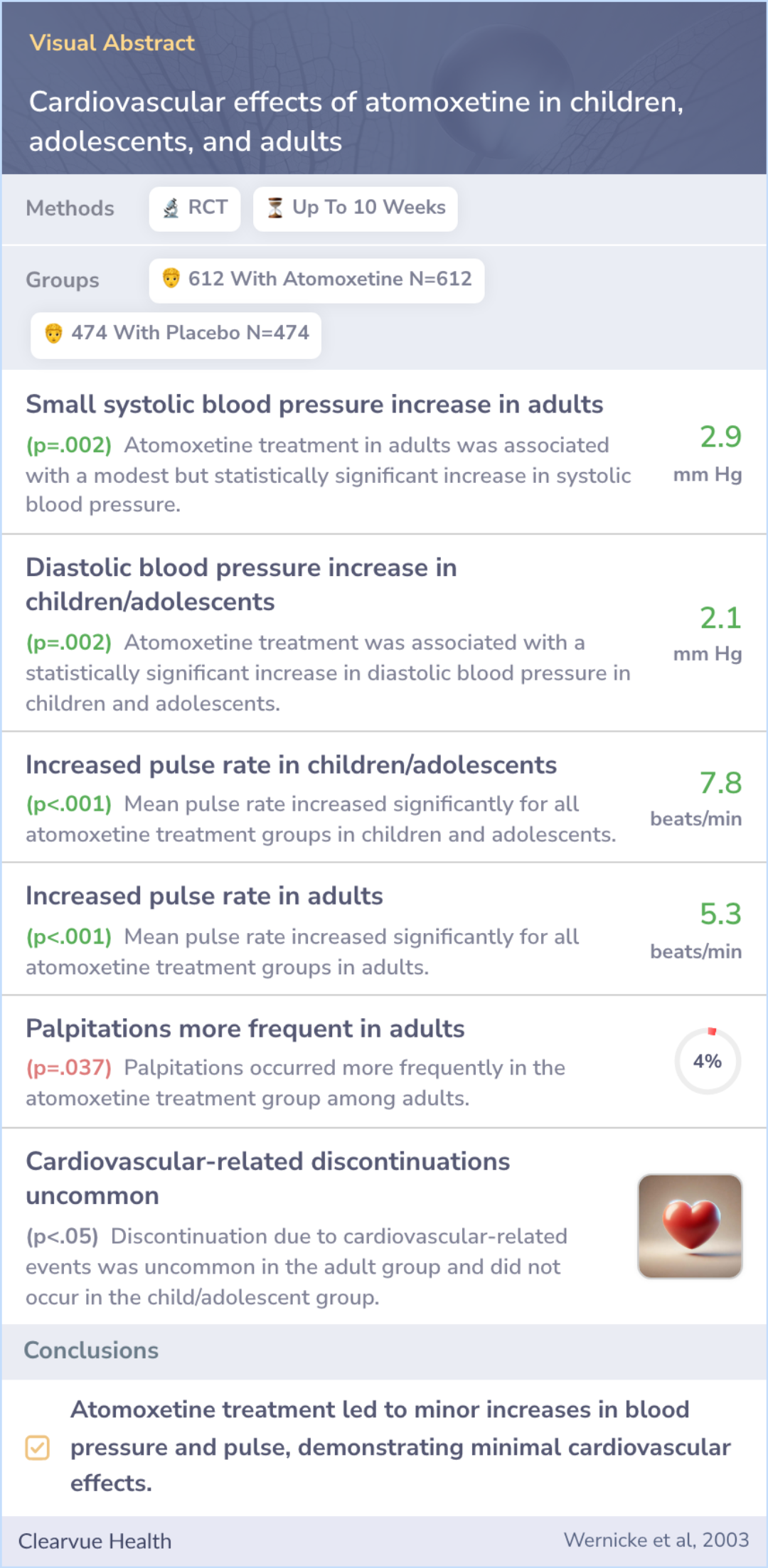
Cardiovascular effects of atomoxetine in children, adolescents, and adults
Cardiovascular effects of atomoxetine
September 14, 2024
author
Wernicke JF, Faries D, Girod D, Brown J, Gao H, Kelsey D, Quintana H, Lipetz R, Michelson D, Heiligenstein J
journal
Drug Saf
Date Published
2003
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
The study examined the cardiovascular effects of atomoxetine, particularly the magnitude and impact of blood pressure and pulse elevations.
💡
What They Found
The study found small but statistically significant increases in blood pressure and pulse rates with atomoxetine, which stabilized and returned to baseline after discontinuation.
📚
What This Means
These findings suggest that, despite small increases in pulse and blood pressure, atomoxetine aligns with current evidence by showing minimal cardiovascular effects and good overall tolerance in short- and long-term use.
Study Summary
Study Overview
This study aimed to explore the cardiovascular safety of atomoxetine, a medication used to treat ADHD. It found that while atomoxetine caused mild increases in heart rate and blood pressure, these changes were generally tolerated well in the short term.
The researchers noted that the long-term effects of these cardiovascular changes are still unclear, especially for patients with pre-existing heart conditions, highlighting the need for ongoing monitoring in this area.
The researchers noted that the long-term effects of these cardiovascular changes are still unclear, especially for patients with pre-existing heart conditions, highlighting the need for ongoing monitoring in this area.
Abstract: background
To further elucidate the magnitude and impact of blood pressure and pulse elevations in patients taking atomoxetine.

Cardiovascular safety concern
"Cardiovascular safety is a significant therapeutic issue in drug product selection."
Potential unknown long-term risks
"The impact of long-term effects is not known, and risks in patients with hypertension or other forms of cardiovascular disease may be different."
Overall cardiovascular toxicity
"Data suggest that atomoxetine is virtually devoid of cardiovascular toxicity during short-term treatment in all patient populations."
Study Summary
Methods
Researchers analyzed the short-term heart health of ADHD patients in trials with atomoxetine (Strattera) or a placebo for up to 10 weeks. In these trials, participants included children, teens, and adults. They also studied long-term effects on children and teens.
Through these trials, doctors checked for blood pressure changes, pulse rates, and potential heart issues using tools like ECGs, which map the heart's activity.
Through these trials, doctors checked for blood pressure changes, pulse rates, and potential heart issues using tools like ECGs, which map the heart's activity.
Abstract: methods
Short-term cardiovascular safety in children, adolescents, and adults with ADHD was assessed in five randomised, double-blind trials (duration up to 10 weeks) with atomoxetine (n = 612) or placebo (n = 474). Long-term cardiovascular safety in childre...more
Study Summary
Results
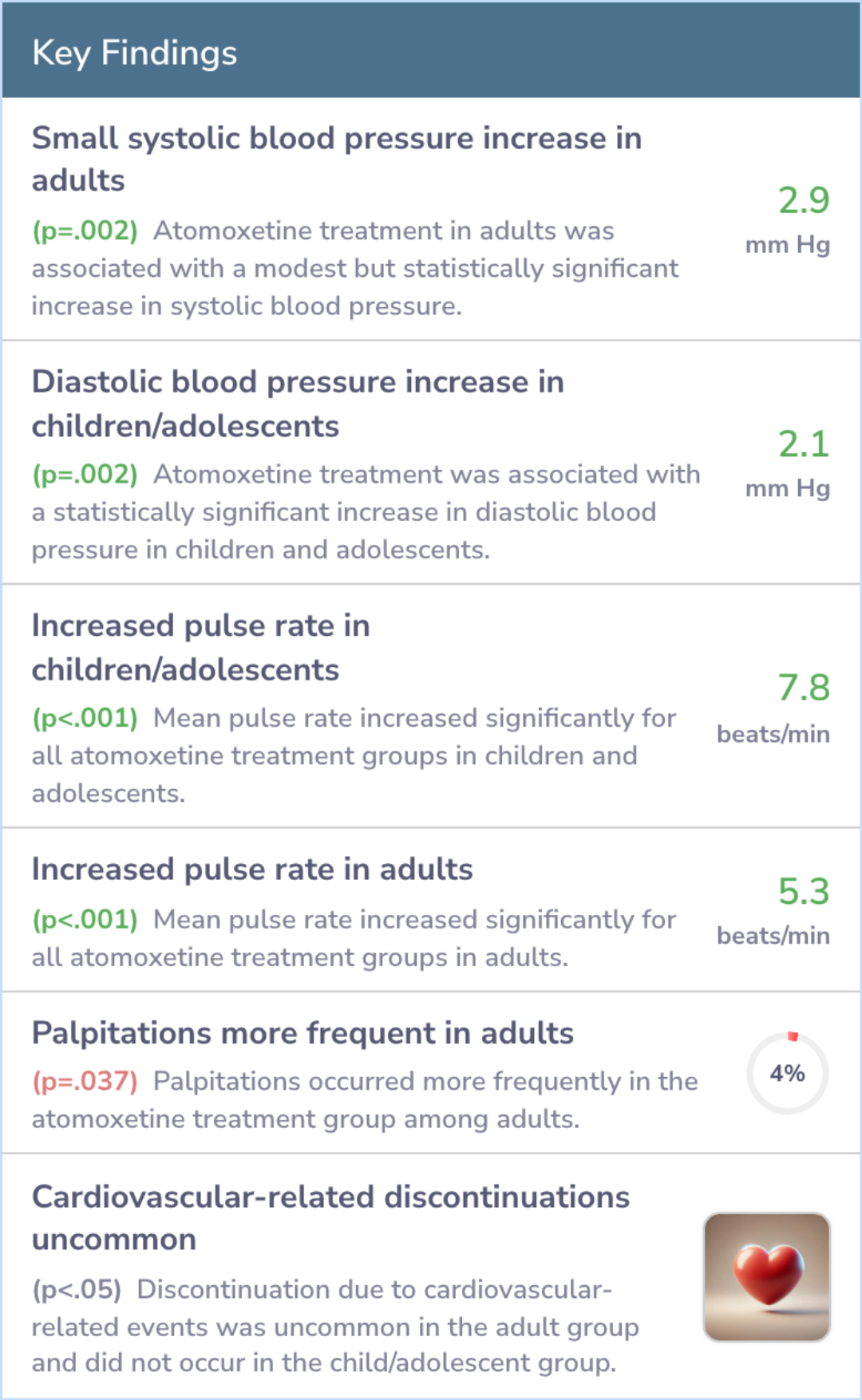
Atomoxetine resulted in a slight but noticeable increase in blood pressure and heart rate, especially at the start; however, these reverted to normal after discontinuation. The change was more in adults' systolic and children's diastolic blood pressure.
Interestingly, there was no difference in heart QT intervals between the medication and placebo. Adults experienced more palpitations with atomoxetine than those on placebo, though treatment cessation due to heart issues was rare.
Interestingly, there was no difference in heart QT intervals between the medication and placebo. Adults experienced more palpitations with atomoxetine than those on placebo, though treatment cessation due to heart issues was rare.
Abstract: results
Atomoxetine treatment was associated with small but statistically significant increases in mean systolic blood pressure in adults and diastolic blood pressure in children and adolescents. Mean pulse rate increased for all atomoxetine treatment groups...more
Study Summary
Conclusions
Despite its effects on nerve signals, atomoxetine caused only small increases in blood pressure and heart rate, likely without significant health concerns. Its effects on the heart's rhythm were negligible.
Overall, the cardiovascular challenges posed by atomoxetine in both short and long-term scenarios were minimal. The medication was generally well accepted among those studied, suggesting its suitability for treating ADHD.
Overall, the cardiovascular challenges posed by atomoxetine in both short and long-term scenarios were minimal. The medication was generally well accepted among those studied, suggesting its suitability for treating ADHD.
Abstract: conclusions
While atomoxetine has noradrenergic activity, increases in pulse and blood pressure were small and of little, if any, clinical significance. Atomoxetine was not associated with QT interval prolongation. Cardiovascular effects of atomoxetine were mini...more

Background Information
Patient Guide
⚙️
Norepinephrine Reuptake Inhibition
Atomoxetine selectively inhibits norepinephrine reuptake, impacting cardiovascular functions.
❤️
Cardiovascular Monitoring Needs
Atomoxetine treatment requires careful monitoring due to potential cardiovascular effects.
🚫
Contraindications for Cardiac Conditions
Patients with serious cardiac issues should avoid atomoxetine due to cardiovascular risks.
⚠️
Adverse Effects and Warnings
Atomoxetine is linked to adverse cardiovascular events; carries warnings for blood pressure monitoring.
💊
Comparison with Other ADHD Medications
Unlike stimulants like methylphenidate, atomoxetine's risk profile for heart issues differs.
Professional Guide
Expert Opinion: Cardiovascular effects of atomoxetine
In light of the observed cardiovascular effects of atomoxetine, close monitoring is a key consideration during treatment.
Monitoring is particularly important due to the potential for increased blood pressure and heart rate.
Atomoxetine administration requires additional caution, especially in patients with pre-existing cardiac conditions or when used with CYP2D6 inhibitors.
Furthermore, its contraindications include serious cardiac anomalies, underlining the importance of assessing patient history prior to prescription.
Monitoring is particularly important due to the potential for increased blood pressure and heart rate.
Atomoxetine administration requires additional caution, especially in patients with pre-existing cardiac conditions or when used with CYP2D6 inhibitors.
Furthermore, its contraindications include serious cardiac anomalies, underlining the importance of assessing patient history prior to prescription.
Evidence Summary
Heart Health Concerns with Methylphenidate Use
Methylphenidate, widely used for ADHD, has drawn attention due to potential heart health concerns. Recent research suggests a possible link between the medication and cardiovascular risks, spurring further investigations.
Patient data shows that those on methylphenidate might face increased risks to heart health, underscoring the need for continued scrutiny into these findings.
Patient data shows that those on methylphenidate might face increased risks to heart health, underscoring the need for continued scrutiny into these findings.
Evidence Summary
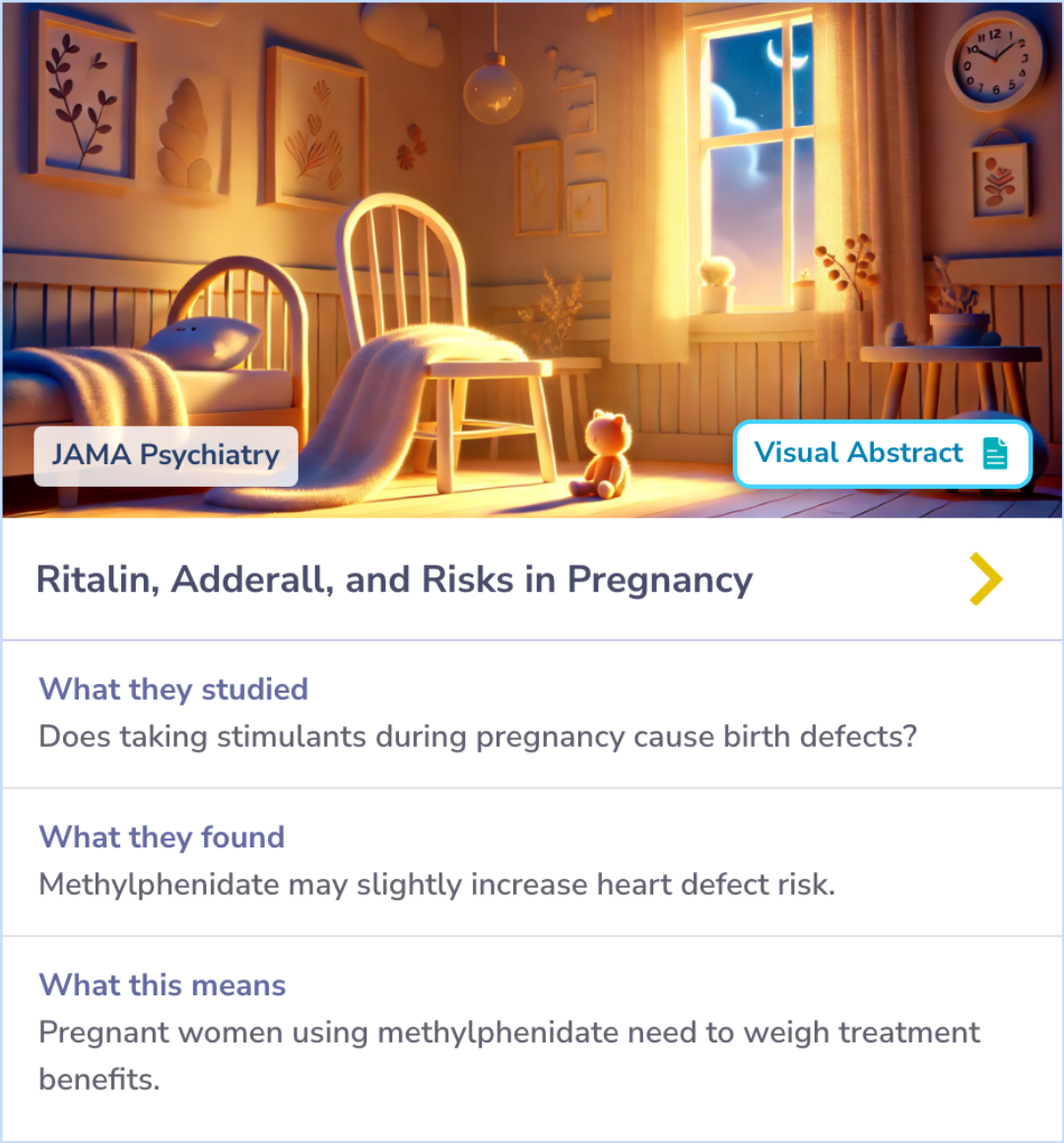
Evaluating Congenital Risks of Stimulant Use in Pregnancy
As stimulant use rises among pregnant women, evaluating its safety becomes crucial. This study explored the risks of congenital malformations linked to first-trimester exposure to methylphenidate and amphetamines. Across large populations in the U.S. and Nordic countries, slight differences emerged between the two, with methylphenidate showing a modest increase in cardiac malformations. However, amphetamines did not show the same risk.
This comparison between stimulants highlights how nuanced and specific the effects can be, especially when considering treatment options for women of reproductive age.
This comparison between stimulants highlights how nuanced and specific the effects can be, especially when considering treatment options for women of reproductive age.
Evidence Summary
Ritalin and Future Substance Risk
Four studies scrutinize the potential link between Ritalin prescriptions and substance abuse. Researchers aim to determine whether those using Ritalin are more susceptible to substance misuse over time. This exploration offers insights into possible long-term effects of Ritalin use.
Findings from these studies contribute to our understanding of risks associated with the medication, highlighting ongoing efforts to unpack the dynamic between Ritalin and substance abuse.
Findings from these studies contribute to our understanding of risks associated with the medication, highlighting ongoing efforts to unpack the dynamic between Ritalin and substance abuse.