Strattera Paper Database
Visual Abstract
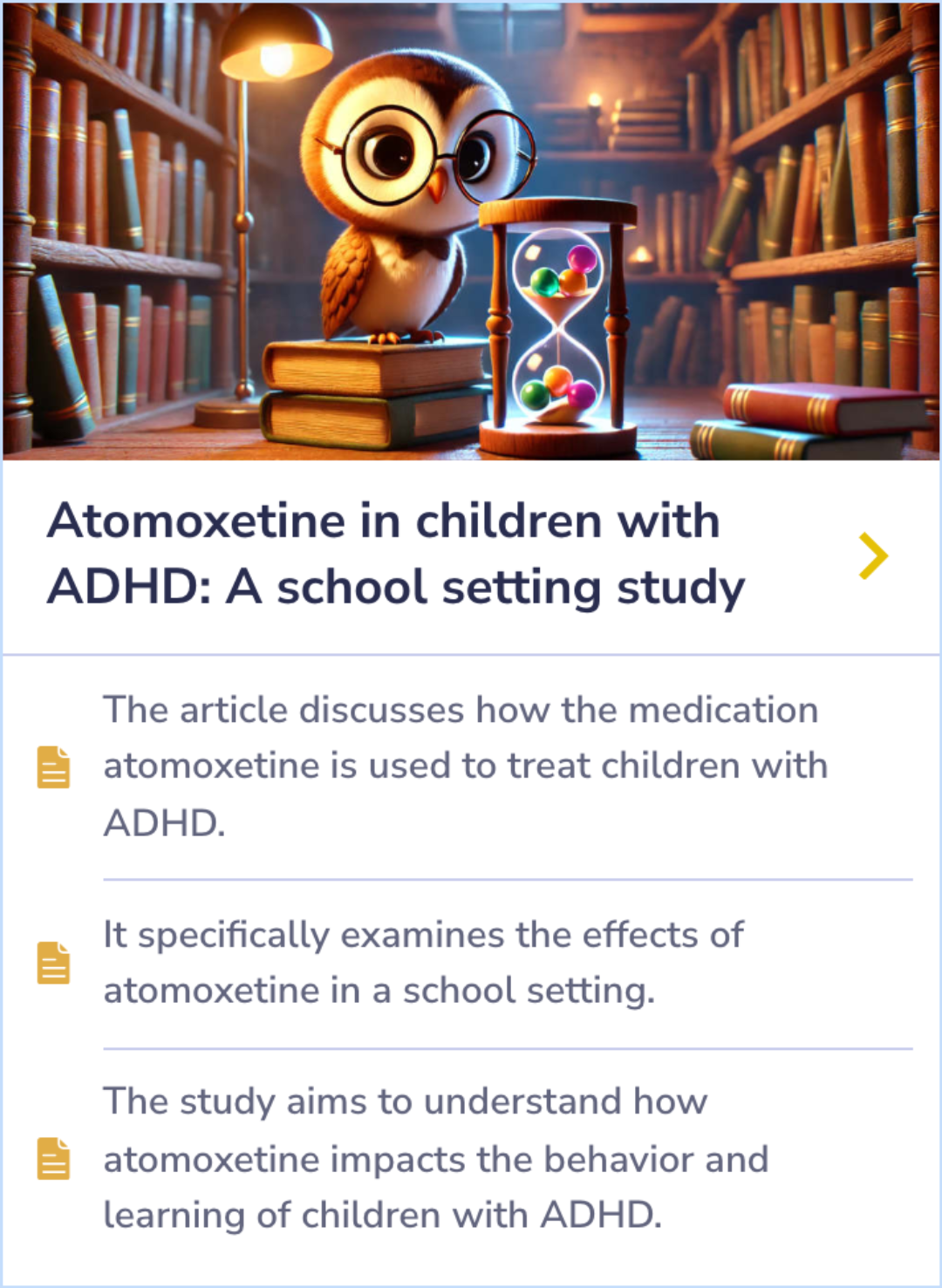
Atomoxetine for the treatment of executive dysfunction in Parkinson's disease: a pilot open-label study
Atomoxetine for Executive Dysfunction in Parkinson's
October 25, 2024
author
Marsh L, Biglan K, Gerstenhaber M, Williams JR
journal
Mov Disord
Date Published
2009-01-30
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
The main research question was whether atomoxetine (Strattera) could improve executive dysfunction in Parkinson's disease patients.
💡
What They Found
The study found that atomoxetine led to improved executive dysfunction in 75% of the patients, with primary outcomes measured by Clinical Global Impression-Change Scale and behavioral scales.
📚
What This Means
Patients with Parkinson's disease experiencing executive dysfunction may see improvement with atomoxetine, aligning with its known benefit in ADHD, but more controlled studies are needed.
Study Summary
Study Overview
This study aimed to explore how atomoxetine might help improve executive dysfunction (ED) in people with Parkinson's disease (PD). Researchers focused on the behavioral aspects of ED to ensure clinical relevance. Early findings showed that atomoxetine was generally well-tolerated and could lead to notable improvements in symptoms, suggesting a possible route for future treatment options.
The implications of this study point to the need for further controlled trials to investigate the effectiveness of atomoxetine for ED in PD, while considering the varied nature of symptoms across individuals.
The implications of this study point to the need for further controlled trials to investigate the effectiveness of atomoxetine for ED in PD, while considering the varied nature of symptoms across individuals.
Abstract: background
Executive dysfunction (ED) is a prominent and often disabling feature of cognitive impairment in Parkinson’s disease (PD). Few studies have examined treatments. Given the role of noradrenergic pathology in ED, atomoxetine, a norepinephrine reuptake i...more

Clinical Relevance
"The focus on behavioral aspects of ED is a unique aspect of this study that ensured its clinical relevance."
Patient Inclusion
"Diagnosis of ED based on clinical symptoms may allow inclusion of patients in trials with disease-related behavioral changes before progression to the point of cognitive deficits on formal testing."
Need for Future Studies
"This pilot study supports the importance of exploring efficacy of atomoxetine for PD-related ED in future studies, using controlled designs."
Study Summary
Methods
The study followed 12 patients who have Parkinson's disease and significant executive dysfunction. They participated in an 8-week trial where they took a flexible dose of atomoxetine, ranging from 25 mg to 100 mg per day.
The design was open-label, meaning both the researchers and the participants knew they were taking atomoxetine. This method aimed to evaluate what dosages were tolerable for the patients and to observe any changes in their cognitive abilities.
The design was open-label, meaning both the researchers and the participants knew they were taking atomoxetine. This method aimed to evaluate what dosages were tolerable for the patients and to observe any changes in their cognitive abilities.
Abstract: methods
12 patients with PD and disabling ED completed an 8-week pilot open-label, flexible dose (25 to 100 mg/day) trial of atomoxetine.
Study Summary
Results
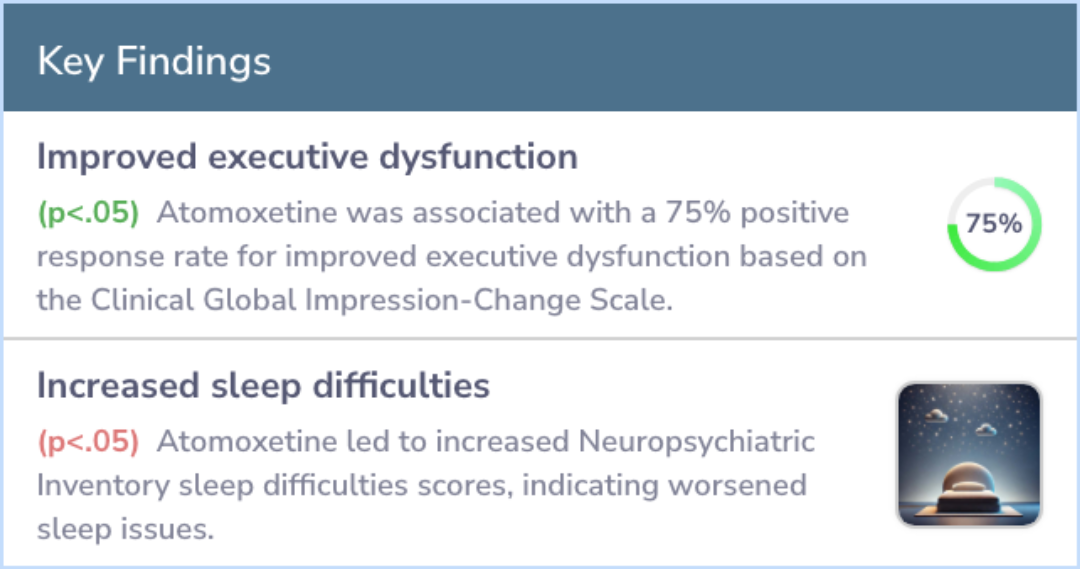
Significant improvements were noticed in the patients' executive dysfunction symptoms. Using the Clinical Global Impression-Change Scale, 75% of the participants showed a positive response. These improvements were also confirmed by two behavioral scales: the Frontal Systems Behavior Scale and the Connors Adult ADHD Rating Scale.
However, some adverse effects were noted, such as sleep problems, gastrointestinal issues, and hypomania. While these side effects were manageable, they warrant closer monitoring in future studies.
However, some adverse effects were noted, such as sleep problems, gastrointestinal issues, and hypomania. While these side effects were manageable, they warrant closer monitoring in future studies.
Abstract: results
On primary outcome measures, atomoxetine was associated with improved ED based on the Clinical Global Impression-Change Scale (75% positive response rate; 95% CI: 43%-95%, p<.05) and behavioral measures of ED [Frontal Systems Behavior Scale (FrSBE) E...more
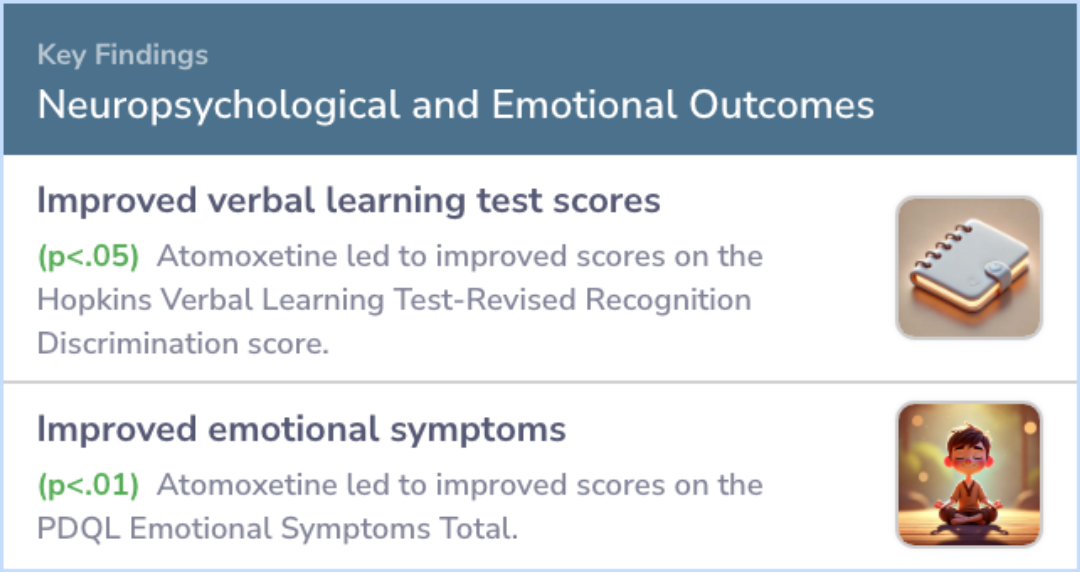
Study Summary
Conclusions
The trial showed that atomoxetine (Strattera) is tolerable for individuals with Parkinson's disease and may help ease their executive dysfunction symptoms. These promising results suggest that the drug could lead to meaningful improvements in daily living activities for these patients.
Given these findings, more extensive, controlled studies are needed to further explore the therapeutic potential of atomoxetine for executive dysfunction in Parkinson's disease. These future trials would help to confirm the preliminary benefits observed and better understand the possible risks.
Given these findings, more extensive, controlled studies are needed to further explore the therapeutic potential of atomoxetine for executive dysfunction in Parkinson's disease. These future trials would help to confirm the preliminary benefits observed and better understand the possible risks.
Abstract: conclusions
Atomoxetine is tolerable in PD and may benefit clinical manifestations of ED, warranting further study in controlled trials.

Background Information
Patient Guide
💊
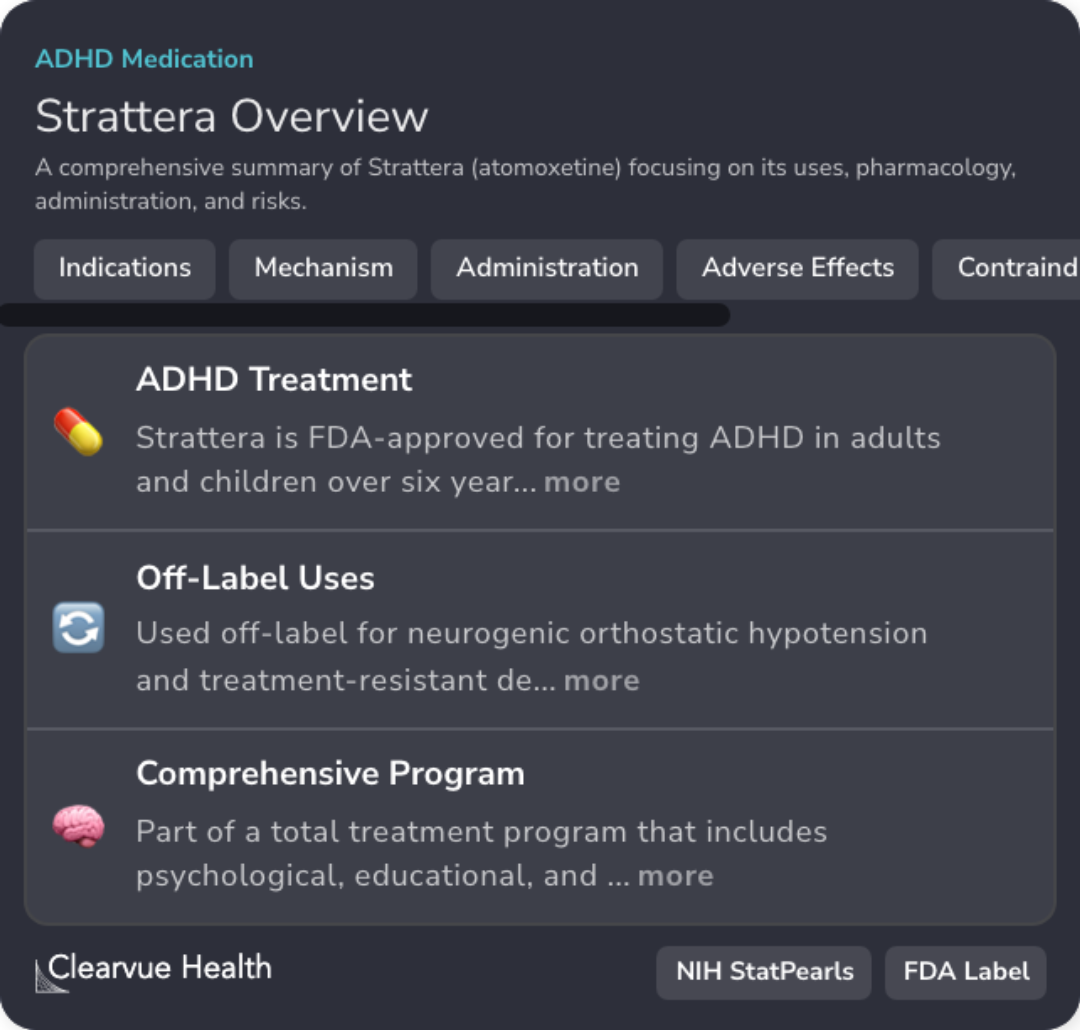
Strattera FDA Approval
Strattera is FDA-approved for treating ADHD in adults and children over six years old.
⚙️
Norepinephrine Inhibition
Atomoxetine functions as a selective norepinephrine reuptake inhibitor, affecting dopamine in specific brain regions.
💊
Dosage and Administration
Atomoxetine is available in 10-100 mg capsules; dosing can be adjusted, with max daily dose of 100 mg.
🤕
Common Adverse Effects
Patients may experience headache, insomnia, nausea, and decreased appetite as common side effects.
⚠️
Potential Serious Risks
Notable risks include suicidal ideation, cardiovascular events, and severe liver injury.
Professional Guide
Expert Opinion: Atomoxetine for Executive Dysfunction in Parkinson's
In line with the abstract's findings on atomoxetine's potential benefits for PD-related executive dysfunction, current professional recommendations highlight its established efficacy in treating ADHD.
However, monitoring for psychiatric effects and potential cardiovascular risks is necessary.
Atomoxetine functions by inhibiting norepinephrine reuptake and is metabolized primarily through CYP2D6, which affects plasma levels.
Adverse reactions may include slowed growth in children, overdosage concerns for poor metabolizers, and serious liver dysfunction symptoms, urging immediate cessation upon their appearance.
However, monitoring for psychiatric effects and potential cardiovascular risks is necessary.
Atomoxetine functions by inhibiting norepinephrine reuptake and is metabolized primarily through CYP2D6, which affects plasma levels.
Adverse reactions may include slowed growth in children, overdosage concerns for poor metabolizers, and serious liver dysfunction symptoms, urging immediate cessation upon their appearance.
Evidence Summary
Atomoxetine's Role in Managing ADHD During School Hours
The study examines the effects of atomoxetine, a medication often used for ADHD, on children's behavior and learning within a school environment. By focusing on how this drug works in a real-world setting, the research sheds light on the potential benefits and challenges of treating ADHD in children during their educational activities.
It highlights how the medication may influence both behavior and academic performance, providing a practical perspective on its use in schools.
It highlights how the medication may influence both behavior and academic performance, providing a practical perspective on its use in schools.
Evidence Summary
Atomoxetine for ADHD in Patients with Tic Disorders
This article dives into how atomoxetine manages ADHD symptoms in patients with tic disorders. It details how the medication helps reduce the symptoms of both ADHD and tics. The text also reviews clinical studies that support using atomoxetine for treating these conditions.
Clinical evidence solidifies atomoxetine's dual role in alleviating ADHD and tic disorders, making it a recommended treatment option.
Insights from several clinical studies offer robust support for atomoxetine's efficacy in these patient groups.
Clinical evidence solidifies atomoxetine's dual role in alleviating ADHD and tic disorders, making it a recommended treatment option.
Insights from several clinical studies offer robust support for atomoxetine's efficacy in these patient groups.
Evidence Summary
Comparing Methylphenidate and Atomoxetine for ADHD Management
The article compares methylphenidate and atomoxetine, focusing on their effectiveness in managing ADHD symptoms. Both drugs are used to help individuals cope with ADHD, and the discussion emphasizes their roles and differences in treatment effectiveness.
It provides a direct comparison showing which medication might be more beneficial for certain individuals, offering insights into their respective merits and how they can aid in managing ADHD symptoms.
It provides a direct comparison showing which medication might be more beneficial for certain individuals, offering insights into their respective merits and how they can aid in managing ADHD symptoms.