Strattera Paper Database
Visual Abstract
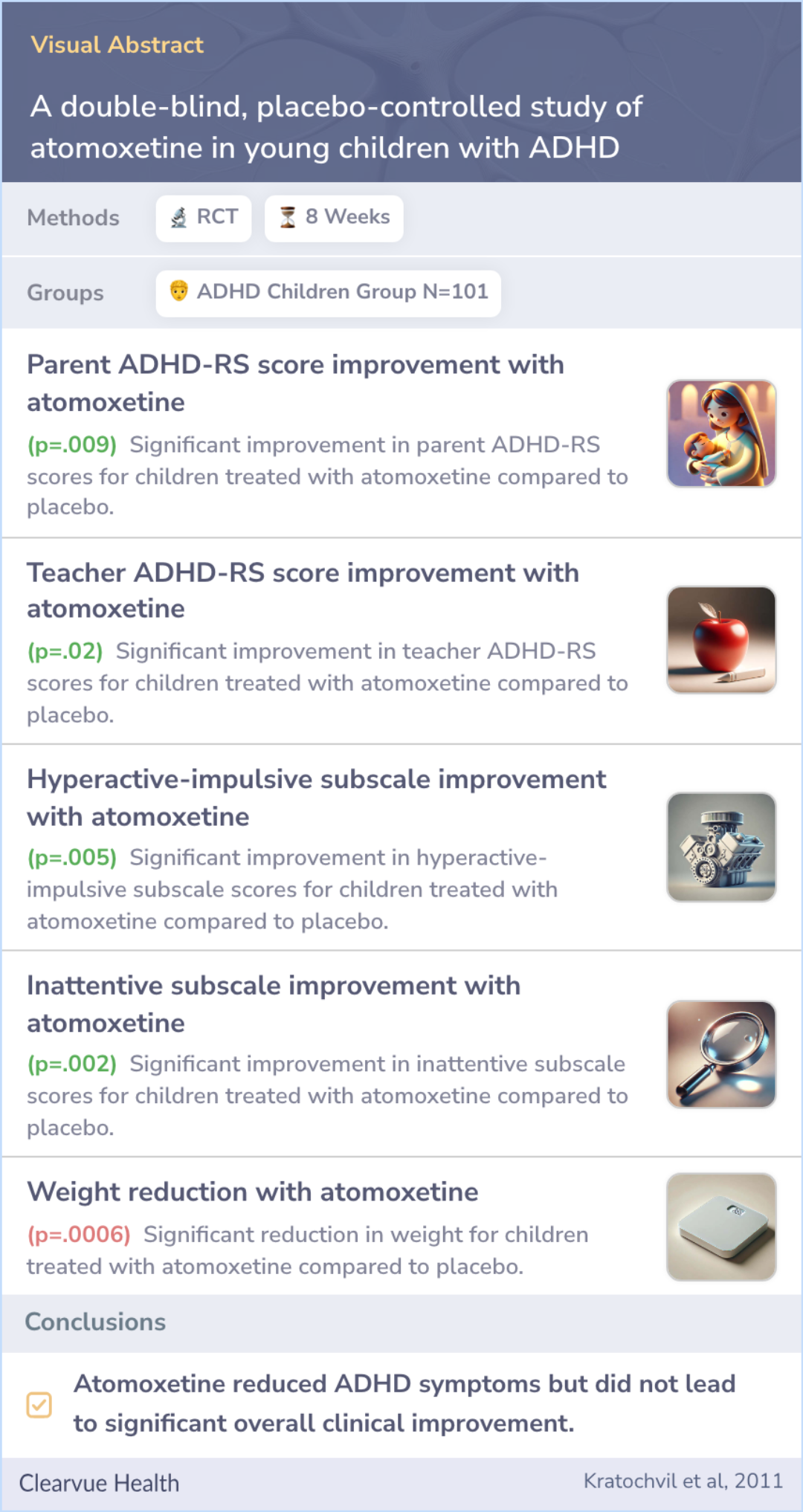
A double-blind, placebo-controlled study of atomoxetine in young children with ADHD
Atomoxetine for ADHD in Young Children
October 25, 2024
author
Kratochvil CJ, Vaughan BS, Stoner JA, Daughton JM, Lubberstedt BD, Murray DW, Chrisman AK, Faircloth MA, Itchon-Ramos NB, Kollins SH, Maayan LA, Greenhill LL, Kotler LA, Fried J, March JS
journal
Pediatrics
Date Published
2011 Apr
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
The study aimed to evaluate the efficacy and tolerability of atomoxetine for treating ADHD in 5- and 6-year-old children.
💡
What They Found
Atomoxetine was shown to significantly reduce ADHD symptoms according to parent and teacher reports, but overall improvement was limited based on clinical impression scales.
📚
What This Means
These findings suggest that while atomoxetine can reduce core ADHD symptoms, it may not lead to significant overall improvement in young children.
Study Summary
Study Overview
The study aimed to assess how effective atomoxetine is for treating ADHD in young children aged 5 and 6. It uniquely included a parent-education module alongside medication to ensure a more comprehensive approach.
While some children showed significant improvements, many still experienced notable ADHD symptoms by the study's end. This highlights the complexity of treating ADHD, especially in very young children, and suggests that more research is needed to explore long-term effects and responses to treatment.
While some children showed significant improvements, many still experienced notable ADHD symptoms by the study's end. This highlights the complexity of treating ADHD, especially in very young children, and suggests that more research is needed to explore long-term effects and responses to treatment.
Abstract: background
To evaluate the efficacy and tolerability of atomoxetine for the treatment of attention-deficit/hyperactivity disorder (ADHD) in 5- and 6-year-old children.

Objective of the Study
"The objective of this study was to evaluate the efficacy and tolerability of atomoxetine for the treatment of ADHD in 5- and 6-year-old children."
Significance of Symptoms
"Although effective, clinically significant symptoms remained for the majority of children treated with atomoxetine."
First Assessment in Young Children
"This study provides the first randomized controlled data from children under the age of 6 years."
Study Summary
Methods
An 8-week clinical trial was conducted where neither the participants nor the researchers knew who received the active drug, atomoxetine, or a placebo. This approach is called double-blind.
During the study, doctors adjusted the medication dose as needed, up to a certain limit. They also provided parents with educational materials on managing ADHD symptoms and behaviors effectively.
During the study, doctors adjusted the medication dose as needed, up to a certain limit. They also provided parents with educational materials on managing ADHD symptoms and behaviors effectively.
Abstract: methods
This was an 8-week, double-blind, placebo-controlled randomized clinical trial of atomoxetine in 101 children with ADHD. Atomoxetine or placebo was flexibly titrated to a maximum dose of 1.8 mg/kg per day. The pharmacotherapist reviewed psychoeducati...more

Study Summary
Results
Atomoxetine led to notable improvements in ADHD symptoms, as reported by parents and teachers. However, only 40% of children on atomoxetine showed significant improvement, compared to 22% on placebo.
Side effects like decreased appetite, stomach issues, and drowsiness were more common with atomoxetine. While some children responded well, others showed less change, and still, many were moderately or severely affected by ADHD symptoms.
Side effects like decreased appetite, stomach issues, and drowsiness were more common with atomoxetine. While some children responded well, others showed less change, and still, many were moderately or severely affected by ADHD symptoms.
Abstract: results
Significant mean decreases in parent (P = .009) and teacher (P = .02) ADHD–IV Rating Scale scores were demonstrated with atomoxetine compared with placebo. A total of 40% of children treated with atomoxetine met response criteria (Clinical Global Imp...more

Study Summary
Conclusions
This study was groundbreaking, being the first to test atomoxetine in children as young as five years. The medication was mostly well-tolerated and helped reduce key ADHD symptoms.
Yet, despite some symptom improvements, many children remained significantly affected by their ADHD at the study's conclusion, highlighting that symptom reduction didn’t always equate to overall functional improvement.
Yet, despite some symptom improvements, many children remained significantly affected by their ADHD at the study's conclusion, highlighting that symptom reduction didn’t always equate to overall functional improvement.
Abstract: conclusions
To our knowledge, this is the first randomized controlled trial of atomoxetine in children as young as 5 years. Atomoxetine generally was well tolerated and reduced core ADHD symptoms in the children on the basis of parent and teacher reports. Reduct...more

Background Information
Patient Guide
💊
ADHD Treatment Authorization
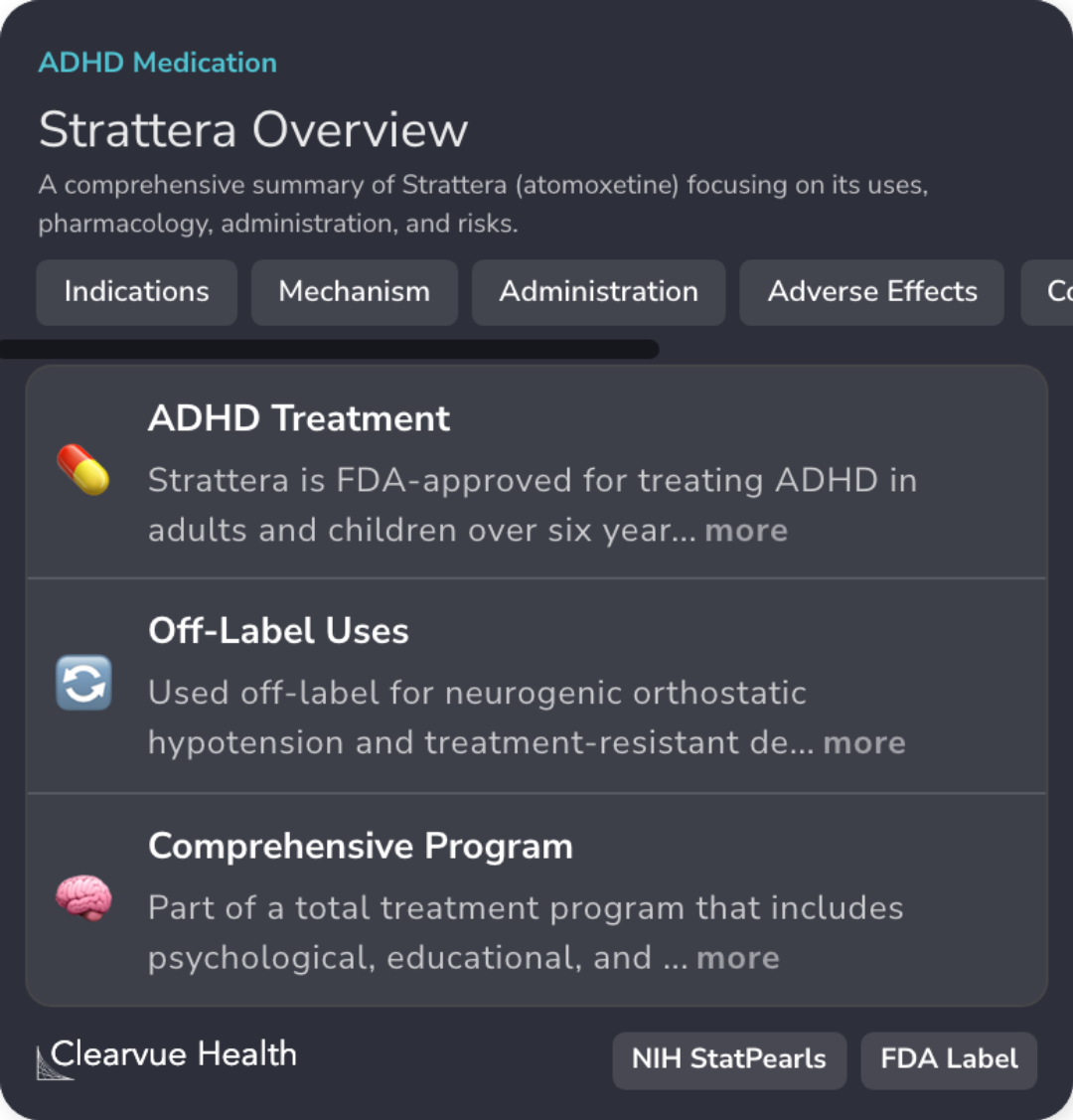
Strattera is FDA-approved for ADHD treatment in children over six years old.
⚙️
Selective Norepinephrine Reuptake Inhibition
Atomoxetine selectively inhibits norepinephrine reuptake, influencing certain brain regions.
🧠
Comprehensive Treatment Strategy
Atomoxetine is part of a program integrating psychological, educational, and social interventions.
📈
Monitoring and Dosing Adjustments
Atomoxetine dosing requires adjustments for CYP2D6 poor metabolizers and specific monitoring.
🤕
Significant Adverse Effects
Common atomoxetine side effects include headache, insomnia, nausea, and decreased appetite.
Professional Guide
Expert Opinion: Atomoxetine for ADHD in Young Children
In line with the abstract findings, atomoxetine shows efficacy in reducing core symptoms of ADHD but may offer a less robust effect compared to stimulant medications.
The guidelines suggest methylphenidate as an alternative for severe symptoms if behavioral interventions fall short.
Given atomoxetine's lower abuse potential compared to stimulants, it remains a viable option, despite the need for careful monitoring for suicidal ideation in children.
Preschool-aged children are advised to commence treatment with evidence-based behavioral interventions.
The guidelines suggest methylphenidate as an alternative for severe symptoms if behavioral interventions fall short.
Given atomoxetine's lower abuse potential compared to stimulants, it remains a viable option, despite the need for careful monitoring for suicidal ideation in children.
Preschool-aged children are advised to commence treatment with evidence-based behavioral interventions.
Evidence Summary
Comparing Adderall XR and Strattera for ADHD Treatment
Adderall XR and Strattera are both medications prescribed to treat ADHD in school-aged children. Each one works differently, and the article explores how they impact attention and behavior in kids.
It also looks at the side effects of each medication, comparing how children respond to them, helping to illustrate the broader discussion of ADHD treatment options.
It also looks at the side effects of each medication, comparing how children respond to them, helping to illustrate the broader discussion of ADHD treatment options.
Evidence Summary
Atomoxetine's Effects on ADHD in Young Adults
Atomoxetine is discussed as a treatment for ADHD in young adults, focusing on how it improves attention and reduces hyperactivity. The overview emphasizes both the benefits of the medication and its potential side effects, providing a balanced perspective on its use.
The information highlights atomoxetine's role in managing symptoms while acknowledging the need to consider both positive outcomes and possible adverse reactions in treatment decisions.
The information highlights atomoxetine's role in managing symptoms while acknowledging the need to consider both positive outcomes and possible adverse reactions in treatment decisions.
Evidence Summary
Age Matters in Atomoxetine's Effect on ADHD
The article evaluates Atomoxetine's effectiveness for ADHD in children, noting distinct outcomes related to age. It explores how younger and older children respond differently, spotlighting variations in treatment success according to age groups. By highlighting age-related responses, the article reveals how Atomoxetine can affect children at different developmental stages, providing insight into its varying effectiveness.
Evidence Summary
Comparing ADHD Medications: Effectiveness and Tolerability
The article examines various ADHD medications, focusing on their effectiveness and how well patients tolerate them. It offers a systematic comparison of studies, highlighting treatment outcomes and safety.
It also delves into how patients handle side effects, providing insights into the tolerability of different medications. The review consolidates this information to present a clear picture of ADHD treatment safety and effectiveness.
It also delves into how patients handle side effects, providing insights into the tolerability of different medications. The review consolidates this information to present a clear picture of ADHD treatment safety and effectiveness.