Strattera Paper Database
Visual Abstract
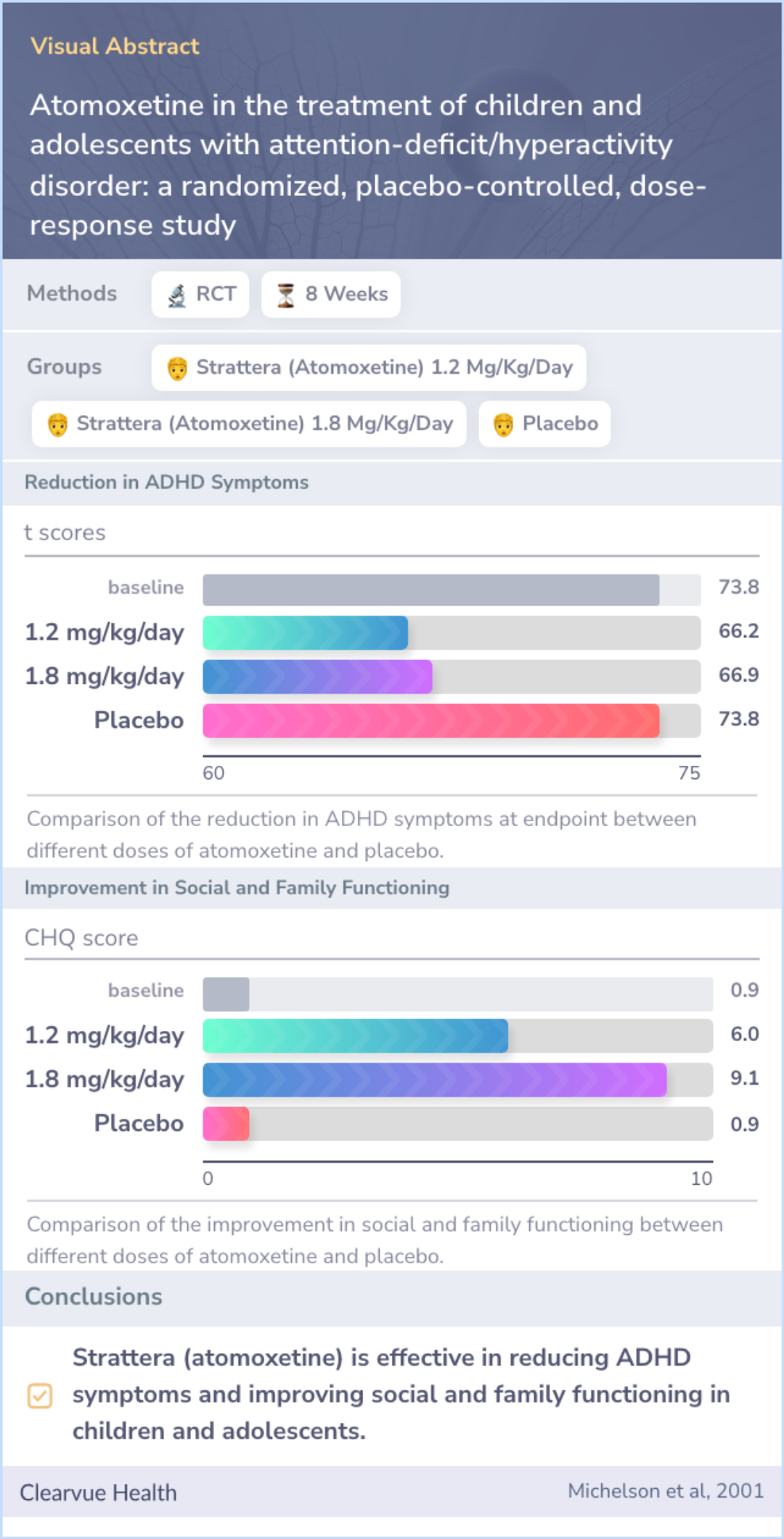
Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study
Atomoxetine for ADHD in Children and Adolescents
October 25, 2024
author
Michelson D, Faries D, Wernicke J, Kelsey D, Kendrick K, Sallee FR, Spencer T
journal
Pediatrics
Date Published
2001 Nov
Why link to a visual abstract?
What is a visual abstract?
Original
Study Summary
🔬
What They Studied
The researchers studied the effectiveness of different doses of Strattera (atomoxetine) compared to a placebo in treating ADHD symptoms in children and adolescents.
💡
What They Found
They found that higher doses of Strattera (atomoxetine) were significantly more effective than placebo in reducing ADHD symptoms and improving social and family functioning.
📚
What This Means
These findings suggest that Strattera (atomoxetine) at doses of 1.2 mg/kg/day is as effective as 1.8 mg/kg/day, providing a strong option for initial treatment in children and adolescents with ADHD.
Study Summary
Study Overview
This study explored how effective atomoxetine is in treating ADHD symptoms in young people. The researchers aimed to find out the best dose that helps the most with managing these symptoms. They discovered that the medication shows strong results and could lead to better daily functioning for kids and their families.
The study also highlighted that atomoxetine is generally safe and well-tolerated, suggesting it can offer meaningful improvements over a significant period.
The study also highlighted that atomoxetine is generally safe and well-tolerated, suggesting it can offer meaningful improvements over a significant period.
Abstract: background
Atomoxetine is an investigational, nonstimulant pharmacotherapy being studied as potential treatment for attention-deficit/hyperactivity disorder (ADHD). It is thought to act via blockade of the presynaptic norepinephrine transporter in the brain. We...more

Efficacy and Dose-Response
"The results of our study provide additional strong evidence of the efficacy of atomoxetine in the treatment of ADHD and extend the findings of the previous report by providing evidence of dose-response and optimal dose, by including adolescents, and by providing additional information about safety and tolerability."
Safety and Tolerability
"The results of this study also provide additional support for the safety and tolerability of atomoxetine. All doses were well tolerated."
Functional Improvement
"These data suggest that atomoxetine's effects on ADHD symptoms were clinically important and associated with a meaningful functional improvement."
Study Summary
Methods
The study involved 297 participants aged 8 to 18, diagnosed with ADHD according to the DSM-IV criteria. Participants were randomly assigned to receive either a placebo or Atomoxetine at doses of 0.5 mg/kg/day, 1.2 mg/kg/day, or 1.8 mg/kg/day.
The trial lasted 8 weeks, during which ADHD symptoms and social functioning were assessed through both parental and investigator ratings.
The trial lasted 8 weeks, during which ADHD symptoms and social functioning were assessed through both parental and investigator ratings.
Abstract: methods
A total of 297 children and adolescents who were 8 to 18 years of age and had ADHD as defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, were randomized to placebo or atomoxetine dosed on a weight-adjusted basis at 0.5...more
Study Summary
Results
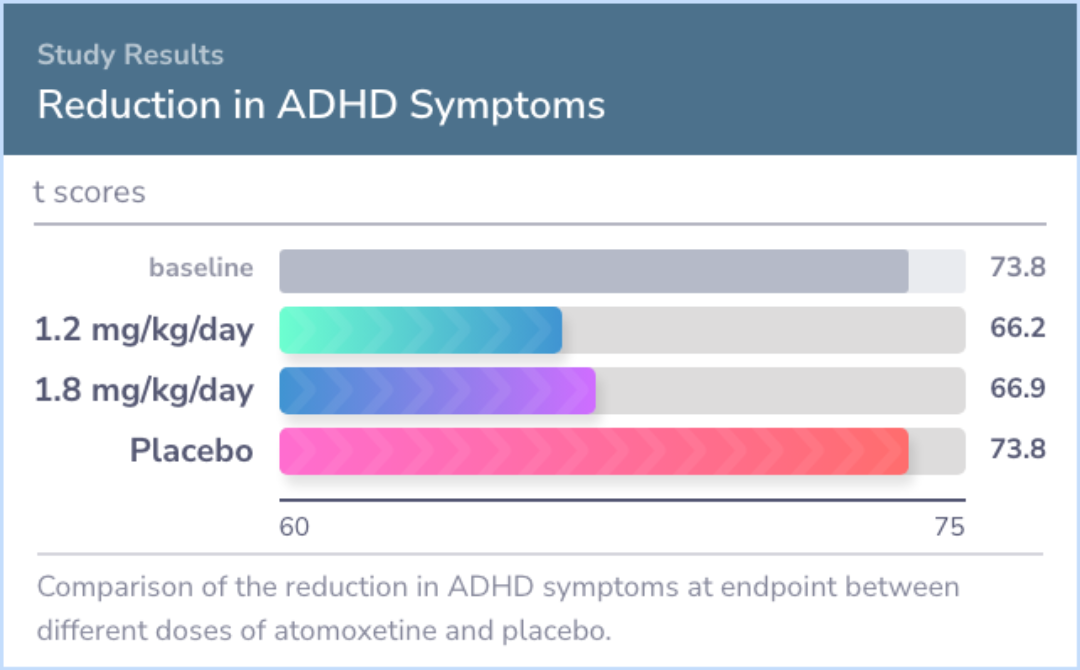
In the trial, about 71% of the participants were male, and 67% displayed mixed ADHD symptoms. Initially, many children had moderate to severe symptoms. By the end, higher doses of Atomoxetine (1.2 and 1.8 mg/kg/day) showed improved ADHD symptoms compared to placebo.
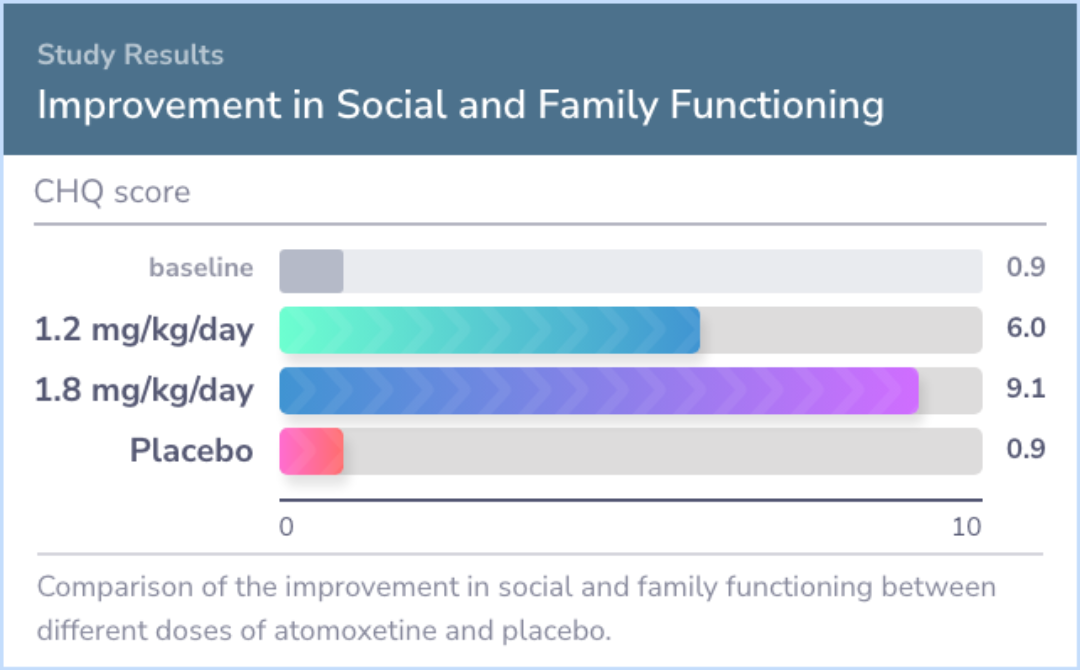
Additionally, the medication positively impacted social and family measures, with less than 5% discontinuing due to adverse events.
Additionally, the medication positively impacted social and family measures, with less than 5% discontinuing due to adverse events.
Abstract: results
Approximately 71% of children enrolled were male, approximately 67% met criteria for mixed subtype (both inattentive and hyperactive/impulsive symptoms), and the only common psychiatric comorbidity was oppositional defiant disorder (approximately 38%...more
Study Summary
Conclusions
For children aged 8 to 18, Atomoxetine was more effective than placebo in treating ADHD and improving social interactions. A dose of 1.2 mg/kg/day is as beneficial as 1.8 mg/kg/day, making it a suitable starting dose for most patients.
Overall, the treatment was deemed safe and well-tolerated, presenting a viable option for managing ADHD symptoms.
Overall, the treatment was deemed safe and well-tolerated, presenting a viable option for managing ADHD symptoms.
Abstract: conclusions
Among children and adolescents aged 8 to 18, atomoxetine was superior to placebo in reducing ADHD symptoms and in improving social and family functioning symptoms. Atomoxetine was associated with a graded dose-response, and 1.2 mg/kg/day seems to be ...more

Background Information
Patient Guide
⚙️
Selective Norepinephrine Inhibitor
Atomoxetine inhibits norepinephrine reuptake, affecting dopamine in specific brain areas.
💊
FDA-Approved for ADHD
Strattera is FDA-approved for ADHD treatment in children above six years.
⚖️
Dosage Adjustments Required
Dosing varies for children based on weight; careful adjustments are needed.
🧠
Comprehensive Treatment Component
Atomoxetine is a part of an ADHD program with psychological and social interventions.
📏
Monitor Growth and Wellbeing
Regular checks for growth and psychiatric symptoms are necessary during treatment.
Professional Guide
Expert Opinion: Atomoxetine for ADHD in Children and Adolescents
In line with the investigated dosing, atomoxetine is indicated for both children and adults over six years.
Experts recommend integrating atomoxetine into a comprehensive ADHD treatment plan to manage symptoms.
Though efficacious, studies show its results may not match those of stimulant medications.
Full therapeutic effects can take several weeks, emphasizing the importance of patience in treatment.
Clinical guidelines caution about a risk of suicidal ideation, necessitating close monitoring.
Common adverse effects, such as insomnia and gastrointestinal issues, also require attention.
Dosage adjustments are essential for patients with hepatic impairment.
Experts recommend integrating atomoxetine into a comprehensive ADHD treatment plan to manage symptoms.
Though efficacious, studies show its results may not match those of stimulant medications.
Full therapeutic effects can take several weeks, emphasizing the importance of patience in treatment.
Clinical guidelines caution about a risk of suicidal ideation, necessitating close monitoring.
Common adverse effects, such as insomnia and gastrointestinal issues, also require attention.
Dosage adjustments are essential for patients with hepatic impairment.
Evidence Summary
Managing ADHD in Children with Atomoxetine
Atomoxetine works by blocking norepinephrine transporters in the brain, which helps manage ADHD symptoms in children. The treatment shows benefits in improving focus and behavior but comes with potential side effects. It's important for caregivers to weigh these benefits against the risks when considering this medication for young patients.
This summary provides a clear look at both the effectiveness and the possible drawbacks of using Atomoxetine in pediatric ADHD treatment.
This summary provides a clear look at both the effectiveness and the possible drawbacks of using Atomoxetine in pediatric ADHD treatment.
Evidence Summary
Atomoxetine's Impact in School Settings
Exploring the use of atomoxetine in classrooms, this piece dives into the medication's effectiveness in managing ADHD symptoms during school activities. With a focus on behavior and learning, the study offers insights into practical outcomes in educational settings.
Attention is paid to how atomoxetine facilitates improved interaction and task completion among students with ADHD, highlighting its role in their daily school life.
Attention is paid to how atomoxetine facilitates improved interaction and task completion among students with ADHD, highlighting its role in their daily school life.
Evidence Summary
Evaluating Long-Term Atomoxetine Use in Teenagers with ADHD
Prolonged use of atomoxetine has shown a significant impact on the symptoms of ADHD in teenagers, emphasizing its role in managing the condition over an extended period. The treatment is carefully evaluated for both safety and benefits, with a particular focus on adolescents undergoing this medication.
The study provides valuable insights into the potential advantages of long-term treatment, especially in improving symptoms and ensuring safety over time.
The study provides valuable insights into the potential advantages of long-term treatment, especially in improving symptoms and ensuring safety over time.